– Synthesize peptides with minimal use of protecting groups, as if connecting blocks –
Researchers) OISAKI Kounosuke, Team Leader, YOSHIDA Masaru, Research Center Director, Functional Group Transformation Team, Interdisciplinary Research Center for Catalytic Chemistry
- Block-by-block peptide synthesis is newly established based on the elongation of peptide chains in opposite direction to conventional peptide syntheses.
- After assembling the peptide chain, the protecting groups are removed all at once, and the complex peptide consisting of nine amino acids are synthesized in large quantities.
- Contributing to mass production of peptides by reducing waste and utilizing inexpensive starting materials
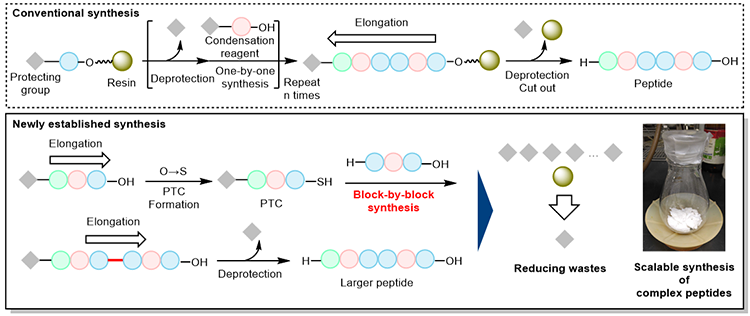
New scalable synthesis of peptides
Researchers at AIST, in collaboration with the University of Tokyo, have realized a low-cost, waste-minimized, scalable chemical synthesis of peptides. Complex peptides consisting of nine amino acids can be produced with minimal use of protecting groups.
To produce peptides in large quantities, "chemical synthesis " is required. Conventional methods elongate amino acids one-by-one in a specific order, but the methods are costly due to requiring expensive amino acid starting materials having protective groups and elaborated chemical activators (condensation reagents). In recent years, chemoselective ligations for unprotected peptide chains have been widely used to efficiently produce large peptides, but most of these ligations have limitations in the scope of amino acid sequences. The newly developed peptide synthesis can be applied to any amino acid sequences, and can connect peptide chains in block-by-block style. By using this method, large peptides can be produced in large quantities with minimal use of protecting groups. As a demonstration of this method, we have succeeded in the scalable synthesis of the bioactive peptide consisting of nine amino acids.
This achievement will contribute not only to the development and supply of new pharmaceuticals (middle-sized molecular drugs), but also to pioneer new applications of peptides, such as industrial uses in foods, agrochemicals, cosmetics, and materials. In addition, synthetic methods that reduce production costs and environmental impact will contribute to the realization of a sustainable society.
A peptide is a chemical compound consisting of several amino acids linked in a chain. Artificial peptides, especially those consisting of 5 to 10 amino acids, recently attracts much attention for application to new pharmaceuticals (middle sized molecular drugs). To synthesize peptides in large quantities, "chemical synthesis" is commonly used.
Conventional chemical synthesis of peptides generally starts with a peptide fragment and elongate the peptide chain by connecting other amino acids one-by-one from a carboxylic acid terminus to amine terminus (C-to-N) direction. However, this method requires the use of numerous "protecting groups" to precisely connect amino acids. Amino acid starting materials having protecting groups are usually expensive, and their use increases production costs. Furthermore, to connect each amino acid, a chemical activator called a "condensation reagent" is required in equal or greater amount than the starting materials. Protecting groups and condensation reagents become waste after the synthesis is complete, increasing the environmental burden.
For these reasons, production of peptides in large quantity with reduced environmental impact and production costs is challenging. In addition, with conventional methods, amino acids can only be connected one-by-one in sequence. If complex peptide fragments could be connected to produce larger peptides, the efficiency of chemical synthesis could be greatly improved. However, such methods are rarely developed because they are prone to cause a side reaction called epimerization, in which stereocenter of the complex peptides alters in the course of reaction, giving an undesired product.
Journal: Communications Chemistry
Title: Practical N-to-C peptide synthesis with minimal protecting groups
Authors: Toshifumi Tatsumi, Koki Sasamoto, Takuya Matsumoto, Ryo Hirano, Kazuki Oikawa, Masato Nakano, Masaru Yoshida, Kounosuke Oisaki, Motomu Kanai
DOI:10.1038/s42004-023-01030-0