- Ongoing evolution of symbiosis observed in natural insect populations across the Japanese archipelago -
Takema Fukatsu (Prime Senior Researcher and Leader of Symbiotic Evolution and Biological Functions Research Group), Takahiro Hosokawa (former AIST Postdoctoral Researcher; currently Assistant Professor of Kyushu University), and members of the Bioproduction Research Institute (BPRI; Director: Tomohiro Tamura), the National Institute of Advanced Industrial Science and Technology (AIST; President: Ryoji Chubachi), in collaboration with the Open University of Japan, University of the Ryukyus, and Okinawa Institute of Science and Technology Graduate University, have discovered that gut symbiotic bacteria of the brown-winged green stinkbug Plautia stali, an agricultural pest that requires gut symbiotic bacteria for its survival, exhibit remarkable geographic variations. All populations in Hokkaido, Honshu, Shikoku, and Kyushu (the mainland of Japan) have a specific symbiont species, whereas the populations in the southwestern islands of japan are associated with multiple symbiont species sympatrically.
In-depth analysis of the stinkbug populations uncovered that a variety of symbiotic bacteria at different evolutionary stages are coexisting in the southwestern islands: (1) a symbiotic bacterium that is uncultivable outside the host body and only vertically transmitted through the host generations; (2) symbiotic bacteria that are cultivable outside the host body, viable in the environment, capable of both vertical transmission and environmental acquisition, and capable of infecting other stinkbug species; and (3) environmental bacteria that are not symbiotic with P. stali in nature but potentially capable of symbiosis upon experimental infections. These results indicate that gut bacterial symbiosis of P. stali in the southwestern islands is not so established as in the mainland, still being in a dynamic evolutionary course.
Thus far, specific processes and mechanisms involved in the establishment of new, sophisticated symbiotic associations have been poorly understood. By capturing a snapshot of ongoing evolution of symbiosis in nature, this research contributes to a better understanding of the origin and evolution of symbiosis, as well as an understanding and control of the adaptation mechanisms of insect pests to the environment.
Details of the results will be published online in an international scientific journal, Nature Microbiology, at 23:00 on January 11, 2016 (Japan Time).
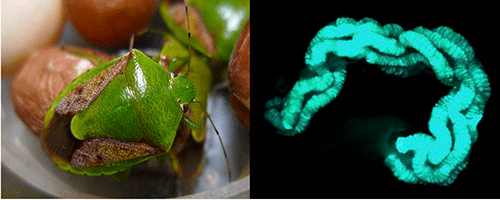 |
Figure: A brown-winged green stinkbug Plautia stali (left) and its midgut symbiotic organ (right)
The blue-green color (right) indicates gut symbiotic bacteria that are localized in the dissected gut. |
The alimentary tract of diverse animals, including humans, harbor a gut microbiota, which consists of a variety of bacteria, fungi and protists, and plays various biological functions. Recently, a series of interesting studies has been published one after another, in which gut microbiota is shown to be substantially relevant to health, disease, and obesity in humans, thereby triggering a significant expansion of researches on the diversity, function, and origin of gut microbiota.
More than 40,000 species of stinkbugs are known from the world, of which over 1,500 species are found in Japan. Since many stinkbugs are agricultural pests, in-depth elucidation of their biological functions is needed from the viewpoint of agriculture and the economy. The stinkbug’s posterior region of the midgut is specialized as a symbiotic organ, which contains specific symbiotic bacteria (often consisting of a single bacterial species) that are essential for the host’s growth and reproduction. These bacteria supply nutrients, such as vitamins and essential amino acids, to the host; at the same time, the bacteria cannot proliferate outside the host body.
Currently, such symbiotic bacteria are highly specialized for sophisticated biological functions. Originally, however, they must have been non-specialized bacteria living in the environment. It has been poorly understood what processes and mechanisms are involved in the evolution of highly specialized symbiotic bacteria from environmental bacteria.
AIST has been making efforts to elucidate the important biological functions of symbiotic bacteria in insects and to understand the sophisticated interactions between insects and symbiotic bacteria. For the gut symbiotic bacteria of stinkbugs that are notorious as agricultural pests, in particular, AIST has made such remarkable achievements as “Symbiotic bacterium can make its host insect pest” (AIST press release on June 13, 2007), “Discovery of symbiotic bacteria mediating insecticide resistance to pest insects” (AIST press release on April 24, 2012), “Novel biological function of polyester in insect-bacterium symbiosis” (AIST press release on June 11, 2013), and “Elucidation of the unique mechanism for symbiont acquisition in stink bug, a group of notorious pest insect” (AIST press release on September 1, 2015). In this research, the researchers discovered the diversity of gut symbiotic bacteria of the agricultural pest P. stali in the Japanese archipelago and attempted to elucidate the process and mechanism by which the diversity has been established.
This research was supported by Grant-in-Aid for Scientific Research (S) by the Japan Society for the Promotion of Science.
The posterior region of the midgut of P. stali (Fig. 1A) is specialized as a symbiotic organ (Fig. 1B), which contains symbiotic bacteria (symbiont A) that are closely related to Escherichia coli (Fig. 1C). Previous studies showed that this symbiont is essential for nymphal growth; upon oviposition, females smear a symbiont-containing excretion onto the egg surface and hatched newborns take it in, which establishes vertical symbiont transmission (Fig. 1D).
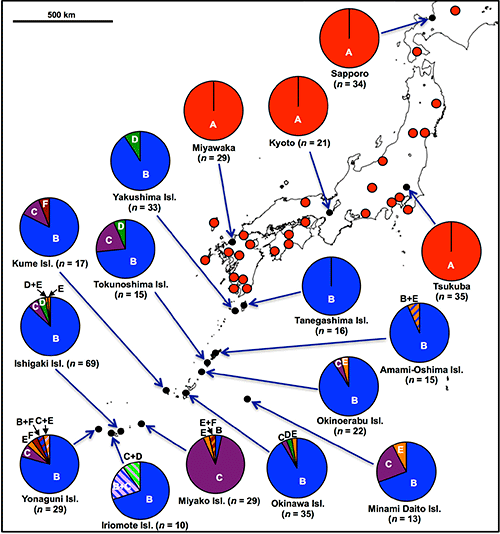
In the previous studies, only P. stali in the mainland had been examined. In this research, the researchers collected 448 insects of P. stali from 42 populations across the Japanese archipelago to investigate their gut symbiotic bacteria, which resulted in the identification of six different gut symbiotic bacteria (symbionts A, B, C, D, E, and F) (Fig. 1E). Of these, only symbiont A was detected in the mainland populations. On the other hand, symbiont B was most frequently detected in the southwestern island populations, whereas symbionts C, D, E, and F were also detected but at lower frequencies (Fig. 2). As an exception, the population of Miyako Island showed that symbiont C infection was dominant.
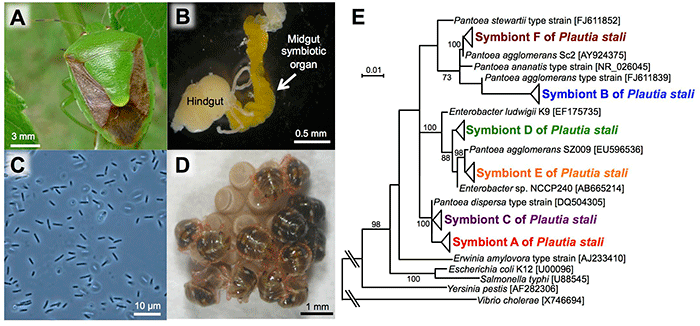 |
Fig. 1: P. stali and its gut symbiotic bacteria
(A) An adult insect. (B) The posterior region of a dissected gut (an arrow indicates the symbiotic organ).
(C) A light microscopic image of symbiotic bacteria. (D) Hatched nymphs acquiring symbiotic bacteria from the egg surface.
(E) Phylogenetic relationship among six species of symbiotic bacteria identified in P. stali populations in Japan (based on 16S ribosomal RNA gene sequences). |
 |
|
Fig. 2: Symbiont polymorphism in P. stali populations in Japan |
When symbionts were removed by egg surface sterilization to prevent vertical transmission to hatched nymphs, most of these symbiont-free offspring died as nymphs. The insects that managed to reach adulthood were dwarf and abnormal in color (Fig. 3). These observations were common when any of symbionts A, B, C, D, E, or F was removed, suggesting that all six species of symbionts are essential for normal nymphal growth.
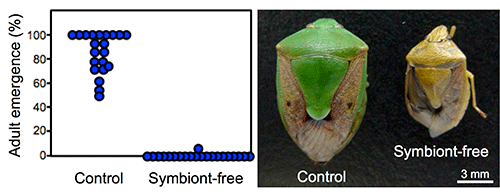 |
Fig. 3: Effects of symbiont elimination on the host stinkbugs
(A) Adult emergence rates. (B) External appearance of the adult insects. |
When symbiotic organs were ground and cultured, no colonies of symbionts A or B were observed, whereas other symbionts (C, D, E, and F) were easily cultured. Draft genome sequencing showed the smaller genome sizes of symbionts A and B compared with those of symbionts C, D, E, and F, suggesting the possibility of genome reduction during the evolutionary course, which may have caused difficulty in the proliferation of the symbionts A and B outside the host body.
The researchers next examined symbiont replaceability. When symbiont B-infected eggs were surface-sterilized and the hatched symbiont-free nymphs were supplied with cultured symbiont C, D, E, or F (Fig. 4A), the growth of these nymphs was similar to that of symbiont B-infected nymphs (Fig. 4B). The same result was obtained in experiments with symbiont A-infected eggs, suggesting that these six species of symbionts play a similar physiological role that is essential for host growth.
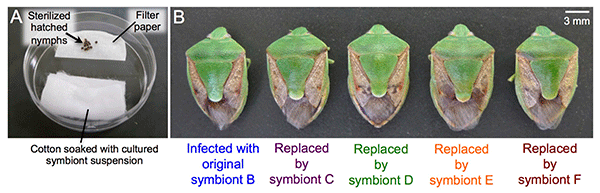 |
Fig. 4: Effects of symbiont replacement on the host stinkbugs
(A) The setting for experimental infection with cultured symbiont. (B) Similar to the original symbiont B-infected stinkbug (left),
the stinkbugs subjected to experimental replacement by symbiont C, D, E, or F (right) exhibited normal growth. |
The finding that symbionts C, D, E, and F are cultivable suggests the possibility that they may be present in the natural environment. To verify this hypothesis, P. stali nymphs hatched from surface-sterilized eggs were exposed for six days to soil samples collected at their habitats on the southwestern Ishigaki Island (Fig. 5A) and subsequently reared with clean food in clean containers. Most of the 1,005 hatched nymphs died, whereas 85 reached adulthood. Of these 85, 14 adult insects were dwarf and abnormal in color, which was typical of symbiont-free insects (Fig. 5C), whereas the remaining 71 were normal in size and color (Figs. 5B and 5D-L). The majority of the normal adults were infected with symbionts C, D, or E; the remaining adults, on the other hand, were infected with previously unknown bacterial lineages (tentatively called environmental bacteria X1-X6) (Fig. 5B-L). These results revealed that cultivable symbionts C, D, and E of P. stali are present in the natural environment and that some other environmental bacteria are potentially capable of symbiosis with P. stali.
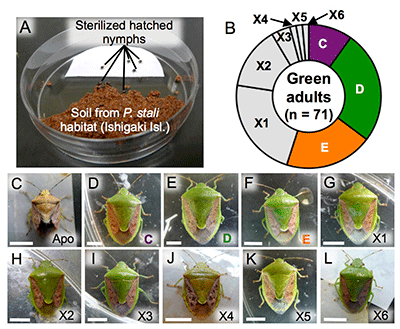 |
Fig. 5: Experimental verification of symbiont acquisition from environmental soil
(A) The setting for experimental infection with soil bacteria. (B) Species of bacteria that infected the green adults.
(C-L) External appearance of the adults infected with the indicated bacterial species. |
The researchers also discovered that many other stinkbug species in the southwestern islands have gut bacteria that are genetically indistinguishable from symbionts C, D, or E of P. stali (Fig. 6A-C). When symbiont B-infected eggs of P. stali were surface-sterilized and the hatched symbiont-free nymphs were supplied with cultured symbiont C, D, or E that had been isolated from symbiotic organs of other stinkbug species, the growth of these nymphs was similar to that of symbiont B-infected nymphs (Fig. 6D). These results suggest that symbionts C, D, and E in the environment may be symbiotic not only with P. stali but also with a variety of other stinkbug species, and that, in addition to vertical transmission from mother to offspring, the symbiotic bacterial community may be associated with the stinkbug community in a dynamic manner, wherein the symbiotic bacteria are often acquired from the environment or moving around between different stinkbug species via horizontal transmission.
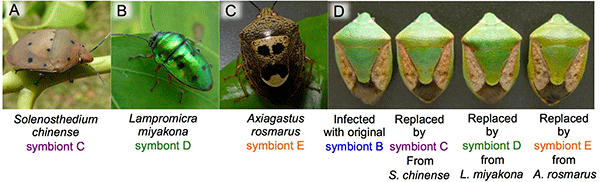 |
Fig. 6: Effects of interspecific symbiont replacement on the host stinkbugs
(A-C) Other stinkbug species harboring gut symbionts that are genetically indistinguishable from symbionts C, D, or E of P. stali.
(D) Similar to the original symbiont B-infected stinkbug (left), the stinkbugs with replaced symbionts C, D, or E derived from other stinkbug species exhibited normal growth. |
As presented above, the gut symbiotic microbiota in P. stali in the Japanese archipelago is in a dynamic evolutionary process. Symbiont A, which cannot survive outside the host body, is completely fixed in the mainland populations of P. stali. On the other hand, the southwestern island populations do not have a fixed symbiont; the majority is symbiont B, which cannot survive outside the host body similar to symbiont A, and, other symbionts C, D, E, and F also coexist. Symbionts C, D, E, and F have properties similar to environmental bacteria, can survive outside the host body, and can infect other stinkbug species. Furthermore, various environmental bacteria were also discovered to be present in environmental soil with the potential of symbiosis with stinkbugs. This research successfully captured the process of ongoing evolution of symbiosis in nature, contributing to a better understanding of the origin and evolutionary process of symbiosis. This research elucidated a new aspect of biodiversity in the southwestern islands where significantly abundant biodiversity is observed.
The researchers plan to monitor the diversity of symbionts over time in natural populations of P. stali in the southwestern islands, and also to promote experimental evolutionary studies in which P. stali gut symbiosis is continuously monitored under various environmental conditions in the laboratory. Of particular interest is elucidating the reason why multiple symbiont species coexist in the southwestern island populations of P. stali, whereas a single symbiont species is fixed in the mainland populations.
In addition, interactions among symbionts, including competition or coexistence, will be analyzed to deepen the understanding of the commonality and diversity of symbiotic mechanisms and to examine the possibility of using symbionts as a new target for controlling stinkbugs as agricultural pests. Symbiont A, which is fixed in the temperate mainland, and symbiont B, which is dominant in the subtropical southwestern islands, may be involved in the adaptation of the stinkbugs to different temperature conditions or different food plants. These issues should be investigated in future studies.