Update(MM/DD/YYYY):11/05/2014
Biological Roles of Symbiont-supplemented Egg-covering Jelly of Urostylidid Stinkbugs
– Secret of nymphs growing in midwinter forest –
Points
-
A voluminous jelly-like substance covering the eggs of urostylidid stinkbugs mainly consists of polysaccharides.
-
The jelly contains nutrients sufficient for growth of nymphs in midwinter as well as essential bacterial symbiont.
-
The discovery of the animal-made agar-like substance is expected to lead to elucidation of its novel physiological functions.
Summary
Takema Fukatsu (Prime Senior Researcher and Leader of Symbiotic Evolution and Biological Functions Research Group), Nahomi Kaiwa (Technological Trainee), and Takahiro Hosokawa (former AIST Postdoctoral Researcher, currently Assistant Professor of Kyusyu University), the Bioproduction Research Institute (Director: Tomohiro Tamura), the National Institute of Advanced Industrial Science and Technology (AIST; President: Ryoji Chubachi), and Shuji Shigenobu (Associate Professor), the National Institute for Basic Biology (NIBB), the National Institutes of Natural Sciences, in collaboration with the Open University of Japan and the University of Tokyo, revealed production mechanisms, chemical constituents, physiological functions, and adaptive significance of the unique egg-covering jelly produced by stinkbugs of the family Urostylididae.
Urostylidid stinkbugs are distributed across Asian regions and countries including Japan. In late autumn, females lay egg masses covered with a jelly-like substance on the trunk of oak trees. The eggs hatch in midwinter, and the nymphs feed solely on the jelly until early spring, when they start to suck plant sap. The jelly, composed of polysaccharides called galactans, contains nutrients sufficient for growth of the nymphs to the third instar, as well as essential bacterial symbiont required for the nymphs that live solely on plant sap from spring. In this way, the jelly supports the peculiar ecology of urostylidid stinkbugs. Massive production of galactans, such as agar, carrageenan and pectin, has been well-known in algae and plants, but exceptional in animals. Elucidation of biological functions of the insect-made galactan gel is of both basic and applied scientific interest.
Details of the results have been published online in an American scientific journal, Current Biology, at 1 a.m. on September 26, 2014 (Japan Time).
 |
|
An adult female urostylidid stinkbug laying an egg mass covered with the jelly-like substance |
Social Background of Research
Insects account for more than a half of all described species, which play substantial roles in terrestrial ecosystems. In order to make use of sophisticated and diverse biological capabilities of this animal group, a variety of research and development has been carried out.
Stinkbugs (Hemiptera: Heteroptera) embrace over 40,000 species in the world and more than 1,500 species in Japan, which contain a number of notorious crop pests and thus are regarded as an agriculturally and economically important insect group. Plant-sucking stinkbugs are generally associated with symbiotic bacteria within the gut, which are essential for survival of the host insects and involved in important biological functions such as provisioning of nutrients, plant adaptation and insecticide resistance.
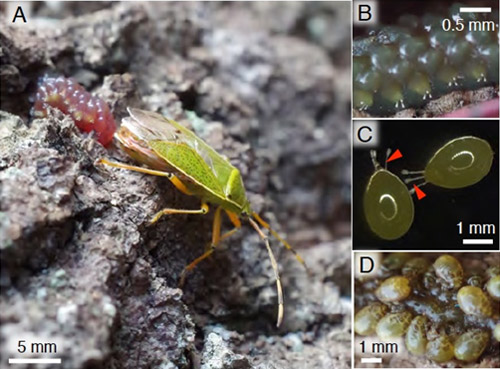
Stinkbugs of the family Urostylididae consist of about 7 genera and 80 species in Southeast Asia and 2 genera and 5 species in Japan. Adult females of Urostylis westwoodii and Urostylis annulicornis in this family, whose abdomen is conspicuously enlarged in late autumn around November, gather on the trunk of oak trees Quercus acutissima and Quercus serrata, and lay egg masses that are covered with the jelly-like substance (Fig. 1A). Eggs with breathing tubes are arranged in two rows and embedded in the jelly (Figs. 1B and C). The eggs hatch in midwinter around February, and the nymphs feed solely on the jelly and grow to the third instar. Then, they start sucking plant sap in late March to early April when the host trees start shooting buds (Fig. 1D). This peculiar ecology of the urostylidid stinkbugs was first reported in a Japanese literature in the 1910’s, and has been recognized by some amateur entomologists and natural photographers. However, no scientific research has been conducted on this subject for approximately 100 years since the first report.
 |
|
Figure 1 : The egg mass of the urostylidid stinkbug
(A) An adult female laying an egg mass covered with the jelly-like substance on tree bark.
(B) An enlarged image of an egg mass. Breathing tubes are protruding from the jelly layer.
(C) Isolated eggs. Arrowheads indicate breathing tubes.
(D) First-instar nymphs gathering on the jelly.
|
History of Research
AIST has achieved a number of world-leading research results in elucidating important biological functions of symbiotic microorganisms in insects (AIST press releases on March 26, 2004, June 13, 2007, December 22, 2009, April 24, 2012, and July 1, 2014) and understanding sophisticated interactions between insects and symbiotic microorganisms (AIST research results announced on July 2, 2007 and May 28, 2012, and AIST press releases on October 29, 2002, June 11, 2013, and June 21, 2013).
For identification of functional molecules produced by insects, in particular, AIST has achieved such remarkable results as “the venomous protease of aphid soldier” (AIST press release on July 27, 2004) and “elucidation of the body color change mechanism in red dragonflies” (AIST press release on July 10, 2012).
NIBB possesses the latest-model next-generation DNA sequencers at the Functional Genomics Facility, and has a number of achievements in the genomics of various organisms including symbiotic bacteria of insects.
This time, the researchers conducted a cooperative research project in order to uncover production mechanisms, chemical constituents, physiological functions, and adaptive significance of the enigmatic egg-covering jelly of urostylidid stinkbugs.
A part of this research was supported by Scientific Research on Innovative Areas and Scientific Research (S) of Grants-in-Aid for Scientific Research by the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Details of Research
Field-collected egg masses of urostylidid stinkbugs were allocated to the following experimental groups: untreated control egg masses (Fig. 2A); jelly-removed egg masses (Fig. 2B); and jelly-restored egg masses, in which the removed jelly was placed back onto the eggs (Fig. 2C). Growth of the nymphs from these egg masses was observed in the laboratory. Although egg-hatching rates among these groups were at the same level (Fig. 2D), the molting rate to the second instar significantly decreased in the jelly-removed group (Fig. 2E), and no nymphs molted to the third instar in this group (Fig. 2F). In addition, the second-instar nymphs of the jelly-removed group were clearly smaller than those of the control group, indicating growth suppression in the absence of the jelly (Fig. 2G). In the jelly-restored group, the molting rates to the second and third instars recovered significantly (Figs. 2E and F). These results suggest that jelly feeding is essential for survival and growth of the urostylidid nymphs.
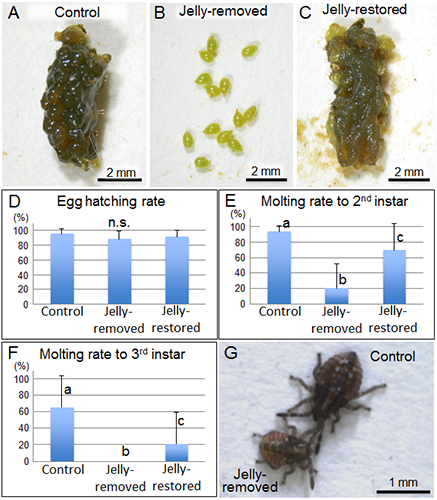 |
Figure 2 : Effects of jelly feeding on nymphs of the urostylidid stinkbug
(A) An untreated control egg mass. (B) A jelly-removed egg mass. (C) A jelly-restored egg mass. (D) Effects on egg-hatching rates.
(E) Effects on molting rates to the second instar. (F) Effects on molting rates to the third instar. (G) Comparison of body sizes of second-instar nymphs.
Different lowercase letters in the graphs indicate statistically significant differences. “n.s.” indicates no significant difference. |
Chemical analysis of the jelly revealed its composition as 60 % water, 26 % carbohydrates (sugars), and 8 % proteins (amino acids). Sugar composition analysis by hydrolyzing the carbohydrates identified galactose as more than 90 % of total sugars, indicating that the jelly is made of polysaccharides mainly consisting of galactose, so-called galactans. Agar and carrageenan from algae and pectin from plants are well-known galactans widely utilized for industrial and commercial purposes. Meanwhile, animal-derived galactans have been poorly described, suggesting the possibility that the jelly may represent a novel biopolymer. Notably, jelly-restored egg masses often grew mold, which resulted in lower molting rates to the second and third instars compared to those of the control egg masses (Figs. 2E and F). These observations suggest the possibility that the outer layer of the jelly may have some antimicrobial activities, which is to be investigated in the future.
Furthermore, the amino acid compositions and contents were analyzed for hydrolyzed eggs, jelly, and third-instar nymphs. The results indicate that the jelly contains sufficient amounts of amino acids for newborn nymphs to grow to the third instar.
Taken together, the egg-covering jelly contains sufficient amounts of essential nutrients for supporting the growth of newborn nymphs to the third instar, which underpins the nymphal growth in midwinter. The peculiar ecology of urostylidid stinkbugs – the nymphs grow in winter when natural enemies are scarce, and already-grown nymphs feed on shooting bugs of high nutritional quality in spring – probably has profound adaptive significance for urostylidid stinkbugs.
When adult females with swollen abdomen just before laying eggs were dissected, highly-developed ovaries accumulated translucent jelly as well as mature eggs at the basal region, indicating that the basal ovarial region is the location of jelly production and storage. Microscopic observation of the internal structure of the egg-covering jelly detected many aggregated symbiont clusters (Fig. 3A). In the jelly-feeding nymphs, the symbionts were accumulated in the posterior end of the alimentary canal (Fig. 3B), suggesting that the symbionts were acquired via intake of the jelly.
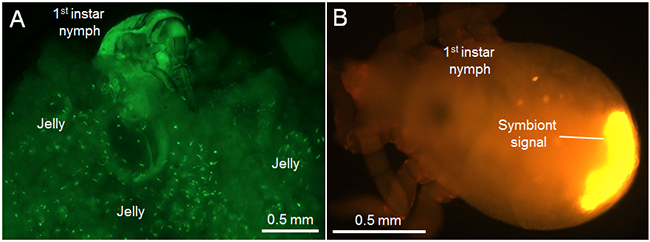 |
Figure 3 : Symbiont acquisition from the egg-covering jelly by nymphs of the urostylidid stinkbug
(A) Small aggregates of symbionts scattered in the jelly. Green granules indicate the aggregated symbionts.
(B) Symbiont localization in the body of first-instar nymph. |
Genome sequencing of the symbiont showed that the symbiont genome retains genes for synthesis of essential amino acids that are required for protein synthesis, although many genes and metabolic pathways are lost and the genome size is extremely reduced. These results suggest that, from spring and on, the symbiont plays an important role for nutritionally supporting the host insect that exclusively lives on plant sap containing little proteins.
Future Plans
The researchers plan to determine the structure of the main jelly components, galactans, and analyze their physical properties and physiological functions. They would also like to examine the potential antimicrobial activities found in the jelly, and causative molecules responsible for the activities. Furthermore, through comprehensive analysis of expressed genes using next-generation DNA sequencing, the researchers will investigate the molecular mechanisms that underlie symbiosis-related functions.