Update(MM/DD/YYYY):09/04/2013
Novel Biological Function of Polyester in Insect-Bacterium Symbiosis
- Unexpected relationship among polyester accumulation, stress resistance, and maintenance of symbiosis -
Points
-
A gut bacterial symbiont of the bean bug accumulates polyester granules under symbiotic conditions.
-
The polyester synthetic ability of the symbiotic bacterium is necessary for the normal symbiotic relationship.
-
This novel finding sheds light on unexpected connections between biotechnology and symbiosis.
Summary
Takema Fukatsu (Prime Senior Researcher and Leader, Symbiotic Evolution and Biological Functions Research Group), Yoshitomo Kikuchi (Senior Researcher, Environmental Biofunction Research Group) and others of the Bioproduction Research Institute (Director :Yoichi Kamagata), the National Institute of Advanced Industrial Science and Technology (AIST; President: Ryoji Chubachi), in collaboration with Pusan National University in South Korea, demonstrated that an intestinal symbiotic bacterium, which has beneficial effects on the growth and reproduction of the bean bug (Riptortus pedestris) known as a soybean pest, accumulates polyhydroxyalkanoate (PHA), a type of polyester, as intracellular granules under symbiotic conditions, and that this intracellular polyester accumulation is required for maintenance of the normal symbiotic relationship.
Many bacteria synthesize large quantities of PHA (sometimes exceeding 90% of bacterial dry weight) from sugars and lipids, accumulating PHA granules within the cell as carbon storage for survival during periods of starvation and environmental stresses. On the other hand, PHA has attracted attention of researchers as a bio-plastic material. AIST’s finding that PHA is involved in the maintenance of the insect-bacterium symbiotic relationship is not only interesting biologically but also sheds light on unexpected connections between biotechnology and symbiosis.
These results have been published online in an American academic journal, Proceedings of the National Academy of Sciences USA, on June 11, 2013 (Japan Standard Time).
 |
|
(Left) The bean bug Riptortus pedestris. (Center) Dissected intestine of R. pedestris, whose posterior is specialized as a symbiotic organ (yellow arrow) for harboring a specific bacterial symbiont. (Right) The symbiont cells wherein accumulated PHA granules are seen (red arrowheads). |
Social Background of Research
Microorganisms are much more diverse than animals and plants, and their sophisticated biological functions have been utilized for a variety of purposes such as producing medical drugs, pesticides, foods, fuels, and detergents, thereby supporting human life and creating huge economic benefits. In recent years, symbiotic bacteria, which live together with animals and plants and exhibit various elaborate biological functions, have attracted much attention as "unexplored bio-resources". Moreover, on the grounds that microbe-derived bio-plastics are recyclable and biodegradable, entails no consumption of fossil fuels in principle, and therefore potentially involves lower environmental loads, not only basic researches on bio-plastics but also application and commercialization of bio-plastics are promoted in the world.
History of Research
AIST has published a number of research achievements in various biological functions of symbiotic bacteria associated with diverse insects, which include symbiont-mediated plant adaption, pest evolution, and insecticide resistance (AIST Press releases on March 25, 2004, June 13, 2007, and April 24, 2012) and in elaborate host-symbiont interactions including nutrient supply, reproductive manipulation, and vertical transmission (AIST Press release on December 22, 2009 and AIST research results announced on July 3, 2007 and May 28, 2012).
Most of symbiotic bacteria have no free-living phase in their life cycle. However, the researchers found that the bean bug (Riptortus pedestris), known as a soybean pest, acquires a bacterial symbiont of the genus Burkholderia from the soil at a nymphal stage and harbors the symbiont in a symbiotic organ located at a posterior region of the digestive tract. The symbiont has beneficial influences on the host fitness including faster growth, larger body size, and more egg production. Furthermore, reflecting its free life in the environmental soil, the Burkholderia symbiont is easily cultivable and genetically manipulatable. Hence the bean bug and Burkholderia comprise a useful model experimental system to elucidate molecular mechanisms underlying symbiosis. AIST focusing on the symbiotic system of the bean bug and Burkholderia, started the international joint research project with the group of Prof. Lee Bok Luel, Pusan National University in South Korea, who is an outstanding expert in the field of insect biochemistry and immunology (AIST TODAY Vol.12 No.4).
This research is supported by the Global Research Laboratory Program of the National Research Foundation of Korea.
Details of Research
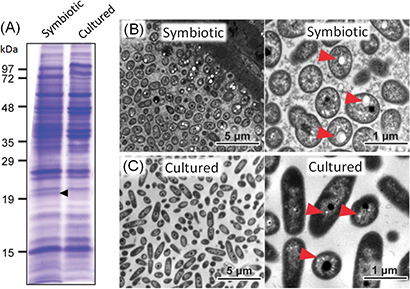
In an attempt to identify molecules involved in the symbiotic relationship between the bean bug and Burkholderia, the researchers prepared symbiotic Burkholderia cells from the host intestine and non-symbiotic Burkholderia cells cultured in a nutrition medium, extracted, analyzed and compared their proteins, and identified a protein over-produced under the symbiotic condition (Fig. 1A). Amino acid sequencing identified the over-expressed protein as PhaP protein that is known to bind to PHA granules. These results suggest that PHA granules are accumulated under the symbiotic condition. Electron microscopy and fluorescent imaging confirmed that symbiotic Burkholderia cells under the symbiotic condition contain well-developed PHA granules (Fig. 1B) whereas cultured Burkholderia cells contain less-developed PHA granules (Fig. 1C).
 |
|
Figure 1 : (A) Comparison of proteins extracted from symbiotic Burkholderia and cultured Burkholderia. A protein band with molecular weight of approximately 19,000 is preferentially detected under the symbiotic condition (black arrowhead). (B) Electron microscopic images of the symbiotic Burkholderia cells wherein well-developed PHA granules are seen (red arrowheads). (C) Electron microscopic images of cultured Burkholderia cells wherein PHA granules are less developed (red arrowheads). |
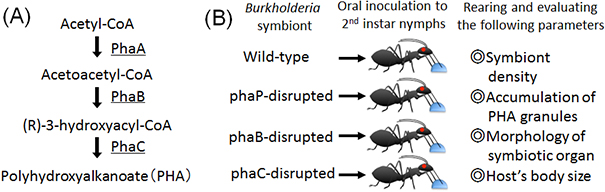
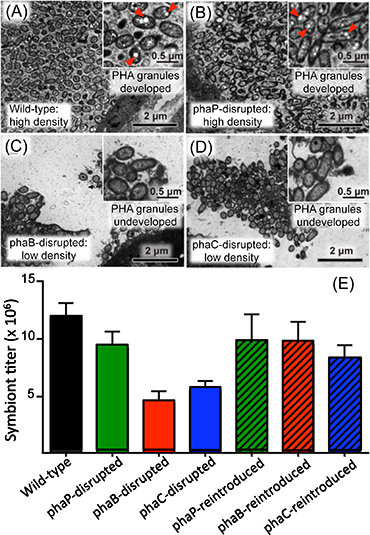
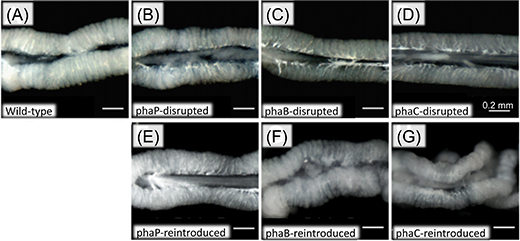
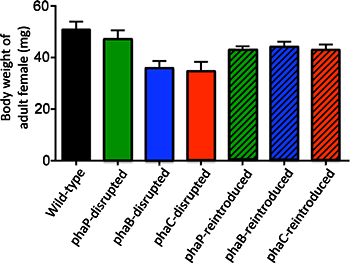
Within bacterial cells, PHA is synthesized by three enzymatic reactions (the enzymes are PhaA, PhaB, and PhaC proteins, respectively) (Fig. 2A). The researchers generated Burkholderia strains with the disruption of phaB gene encoding PhaB protein or phaC gene encoding PhaC protein. The bean bugs were infected with the phaB-disrupted Burkholderia strain, the phaC-disrupted Burkholderia strain, the wild-type Burkholderia strain, or a Burkholderia strain with the disruption of phaP gene encoding PhaP protein that is not involved in the PHA synthesis though it binds to PHA granules (Fig. 2B). In the wild-type strain and the phaP-disrupted strain, normal colonization to the host intestine, normal infection at a high density, and normal accumulation of intracellular PHA granules were observed (Figs. 3A, 3B, and 3E). In the phaB- and phaC-disrupted strains, by contrast, PHA granules were hardly accumulated and the infection density was very low in the host intestine (Figs. 3C-E). Furthermore, the host insects infected with the phaB-disrupted strain or the phaC-disrupted strain exhibited a less developed symbiotic organ and smaller body size than the host insects infected with the wild-type strain or the phaP-disrupted strain (Figs. 4A-D and Fig. 5).
 |
|
Figure 2 : (A) Synthetic pathway of PHA. (B) Experimental design of Burkholderia inoculation test. |
 |
|
Figure 3 : (A)-(D) Comparison of electron microscopic images of wild-type Burkholderia strain and mutant Burkholderia strains in which a PHA-related gene is disrupted. Red arrowheads indicate PHA granules. (E) Comparison of infection densities of wild-type Burkholderia strain, mutant Burkholderia strains with PHA-related gene disruption, and mutant Burkholderia strains to which the disrupted PHA-related gene was reintroduced. |
 |
|
Figure 4 : External appearance of the host symbiotic organs infected with wild-type Burkholderia strain, Burkholderia strains with PHA-related gene disruption, and Burkholderia strains to which the disrupted PHA-related gene was reintroduced. The organ appears swollen and whitish when infection density of the symbiont is high. |
 |
|
Figure 5 : Body weight of the female adult insects infected with wild-type Burkholderia strain, Burkholderia strains with a disrupted PHA-related gene, and Burkholderia strains to which the disrupted PHA-related gene was reintroduced. |
Next, using plasmids encoding the normal phaB or phaC gene, the researchers genetically complemented the phaB- and phaC-disrupted Burkholderia strains, thereby generating the phaB- and phaC-reintroduced Burkholderia strains. Then the host insects were infected with one of these symbiont strains, which also included the wild-type strain containing a blank plasmid and the phaP-reintroduced strain. In the phaB- and phaC-reintroduced strains, accumulation of PHA granules, infection density in the host intestine, development of the host symbiotic organ, and body size of the host insects were all restored to the same levels as those observed with the wild-type strain and the phaP-reintroduced strain (Figs. 3E, 4F, 4G, and 5).
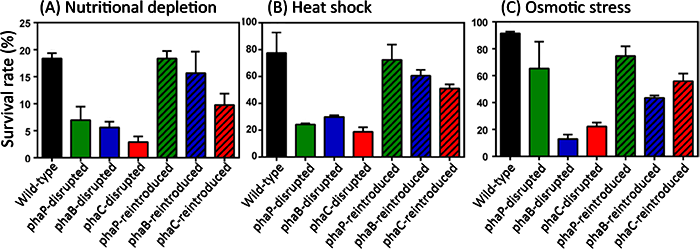
Why does accumulation of PHA granules play an important role in the bean bug-Burkholderia symbiosis? A possible explanation is proposed as “symbiont-mediated stress resistance” hypothesis as follows. Burkholderia cells are harbored in the host symbiotic organ at a very high density (Figs. 1B, 3A, and 3B), and their growth rate in the host body is very slow. Hence, during the host lifetime for about two months, symbiotic Burkholderia might be subjected to environmental stresses caused by high density symbiosis and growth control via, for example, host’s immune regulation. Hence, it is expected that, although speculative, the accumulation of PHA granules may provide the symbiotic Burkholderia with stress resistance. Actually, when experimentally subjected to such environmental stresses as nutritional depletion, high temperature, and high osmotic pressure, the phaB- and phaC-disrupted Burkholderia strains were vulnerable to the stress treatments, while the phaB- and phaC-reintroduced Burkholderia strains were resistant (Fig. 6).
 |
|
Figure 6 : Survival rate of wild-type Burkholderia strain, Burkholderia strains with a disrupted PHA-related gene, and Burkholderia strains to which the disrupted PHA-related gene was reintroduced, under stress conditions. (A) Two days within phosphate buffer solution (starvation). (B) 10 minutes in a 45 ℃ bath (heat shock). (C) 24 hours within 1 mole of glucose solution (hyper-osmotic). |
Future Plans
The researchers are planning to further investigate the mechanisms as to how PHA is associated with the establishment and maintenance of the symbiotic relationship between the bean bug and Burkholderia, by narrowing down the symbiotic stress factors, comprehensive analysis of gene expression, functional analysis, and identification and analysis of symbiosis-related and PHA accumulation-related genes.
This study suggests that the PHA accumulation is involved in bacterial ability of symbiosis, or enduring high density conditions within host organism, which leads to the possibility that, by genetic introduction and/or reinforcement of the PHA synthetic pathway, it may be possible to confer within-host stability and maintenance of bacteria, in particular intestinal ones utilized for probiotic purposes. The researchers are also planning to conduct the research in such an applied perspective.