Haoshen Zhou (Leader), the Energy Interface Technology Group, the Energy Technology Research Institute (Director: Yasuo Hasegawa) of the National Institute of Advanced Industrial Science and Technology (AIST) (President: Hiroyuki Yoshikawa) and Yonggang Wang (Japan Society for the Promotion of Science (JSPS) Postdoctoral Fellow) have developed a new-type lithium-air cell with large-capacity based on new structure.
Lithium-ion batteries are widely used in mobile phones, computers and other electronic devices, however, their energy density is still insufficient for them to be used in electric vehicles. Lithium-air batteries are attractive because they are theoretically advantageous in terms of their potential for future large-capacity batteries. However, one of serious problems with lithium-air batteries reported to date is that a solid reaction product (Li2O or Li2O2), which is not soluble in organic electrolyte, clogs on the air electrode (=cathode) in the discharge process. If the air electrode is fully clogged, O2 from atmosphere cannot be reduced any more.
In this study, an organic electrolyte is used on the anode (metallic lithium) side and an aqueous electrolyte is used on the cathode (air) side. The two electrolytes are separated by a solid state electrolyte (lithium super-ion conductor glass film=LISICON) so that the two electrolytic solutions do not intermix. Only lithium ions pass through the solid electrolyte, and the battery reactions proceed smoothly. At the cathode, the reaction product in the discharge process is water-soluble and no solid substances are produced. Continuous discharging of 50,000 mAh/g (per unit mass of the carbon, catalyst and binder) has been experimentally confirmed.
This technology holds great potential for automobile batteries. At a filling station, the driver of a vehicle thus equipped could exchange the aqueous electrolyte for the air electrode and refill the metallic lithium for the anode in the form of cassettes, and then continue driving without waiting for batteries to be recharged. It is easy to retrieve metallic lithium from the aqueous electrolyte, so that lithium can be reused. This is truly a new type of lithium fuel cell.
The result of the study will be presented at an Electrochemical Society of Japan meeting held in Kyoto on March 31, 2009.
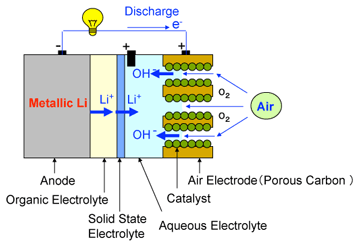 |
 |
Left: Configuration of a newly developed lithium-air cell
Right: Comparison of cathode discharging capacity with that of conventional lithium ion battery.
mAh per 1 gram of cathode
Calculated based on mass of air electrode = (porous carbon + catalyst + binder) and mass of cathode of a lithium-ion battery = (active material + conduction assisting carbon + binder) |
Because of the increase in carbon dioxide emissions caused by the consumption of fossil fuels, as well as the drastic change in oil prices, the shift from gasoline or light oil to electrical energy as an automobile energy source is gaining attention. Electric vehicles have limitation due to the performance of lithium ion battery. The low energy density of lithium ion battery makes the longer distance driving of electric vehicles difficult now.
To popularize electric cars, we need an energy density that is six to seven times greater than those of current lithium ion batteries. Lithium-air batteries are attractive in this regard because they have a very large energy density compared with lithium-ion batteries. In lithium-air batteries, we can use oxygen in air, which is not included in the battery as an active material at the cathode. The capacity of the cathode is infinite in theory, and a large capacity can be achieved.
Targeting high performance lithium-ion batteries, the Energy Technology Research Institute of AIST demonstrated that nano-structured electrode materials provide high rate performance of Lithium ion batteries (AIST press releases of Jan. 18, 2005). Lithium-air batteries also have been aggressively developed to achieve even larger energy densities.
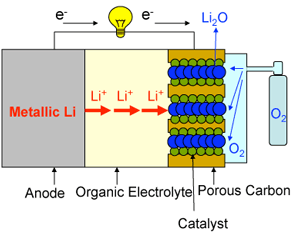 |
Problems with conventional lithium-air cells:
|
1) |
A solid reaction product (Li2O) accumulates at the cathode. It blocks pores, and disturbs discharging. |
|
2) |
Hydrogen gas, which is dangerous, is produced if moisture in the air reacts with metallic lithium. |
|
3) |
Discharging would be disturbed if the nitrogen gas in the air reacts with metallic lithium. |
|
|
Fig. 1 Schematic diagram of a conventional lithium-air (oxygen) battery.
|
To overcome problems with conventional lithium-air batteries, AIST has been developing new lithium-air batteries that use an organic electrolyte and metallic lithium on the anode side, the air and an aqueous electrolyte on the cathode side, and a solid electrolyte LISICON as a partition wall to separate them.
This study was partly supported by Grants-in-Aid for Scientific Research program of JSPS.
This study is based on the idea of using an organic electrolyte only on the anode side, and using an aqueous electrolyte on the cathode side. If the two sides are separated by a solid electrolyte which allows only lithium ions passing through, the two electrolytic solutions do not mix with each other while the charge-discharge reactions proceed smoothly. In this system, it has been confirmed that the discharge reaction product is not a solid substance like lithium oxide (Li2O), but lithium hydroxide (LiOH), which dissolves in the aqueous electrolyte, and so clogging of the pores does not occur at the carbon cathode. Furthermore, as water and nitrogen do not pass through the solid electrolyte (the partition wall), there are no unwanted reactions with the metallic lithium anode. During charging, corrosion and degradation of the air electrode is prevented by using another cathode electrode exclusively for charging.
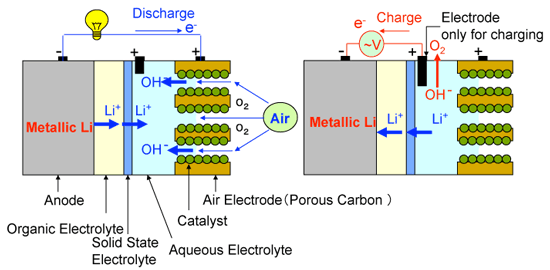 |
|
Fig. 2 Configuration of the new type of lithium-air cell: left, discharging; right, charging |
Metallic lithium is used as the anode, and an organic electrolyte containing lithium salt is used on the anode side. A lithium-ion solid electrolyte is placed in between the two electrolytic solutions as a partition wall to separate the cathode and anode sides. An alkaline water-soluble gel is used as the aqueous electrolyte for the cathode side and the cathode consists of porous carbon and an inexpensive oxide catalyst.
The discharging reactions proceed as follows:
1) Reaction at the anode: Li → Li
+ + e
- Lithium ions dissolve into the organic electrolyte as lithium ions (Li
+) and the electrons are fed into the conductor wire. The dissolved lithium ions (Li
+) pass through the solid electrolyte into the aqueous electrolyte on the cathode side. 2) Reaction at the cathode: O
2 + 2H
2O + 4e
- → 4OH
- Electrons are fed from the conductor wire, and oxygen from the air and the reduction reacts on the surface of catalyst in the porous carbon to produce hydroxyl ions (OH
-). They meet with lithium ions (Li
+) in the aqueous electrolyte and produce water-soluble lithium hydroxide (LiOH).
The charging reactions proceed as follows:
1) Reaction at the anode: Li
+ + e
- → Li Electrons are fed from the conductor wire, and lithium ions (Li
+) in the aqueous electrolyte of the cathode side pass through the solid electrolyte and reach the surface of the anode where metallic lithium precipitates. 2) Reaction at the cathode: 4OH
- → O
2 + 2H
2O + 4e
-
Oxygen gas is generated. Generated electrons are fed to the conductor wire.
The newly developed lithium-air cell with alkaline aqueous electrolyte gel has a discharging capacity of approximately 9000 mAh/g when it is discharged in the air at a discharge rate of 0.1 A/g. The charging capacity is about 9600 mAh/g. These values are considerably larger than the reported values of conventional lithium-air batteries (700 - 3000 mAh/g). Furthermore, by using an alkaline aqueous solution in place of an alkaline water-soluble gel, continuous discharging up to 20 days at the discharge rate of 0.1 A/g in the air has been realized. The discharge capacity of the cell was approximately 50,000 mAh/g (shown in Fig. 3).
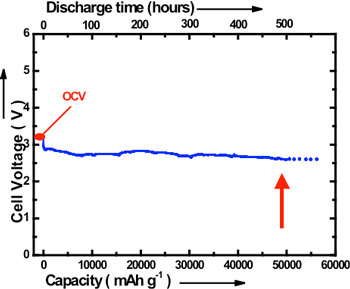 |
|
Fig. 3 Long-term discharge curve of the newly developed lithium-air cell |
The new lithium-air batteries allow for continuous operation if the aqueous electrolyte on the cathode side is exchanged and metallic lithium is resupplied to the anode by means of cassettes, etc. This concept can be taken as a new type fuel cell, called a "lithium fuel cell." By retrieving LiOH from the aqueous electrolyte in the air electrode, metallic lithium can be recovered easily and reused as fuel. Figure 4 shows the concept of this new lithium fuel cell in which lithium is used cyclically.
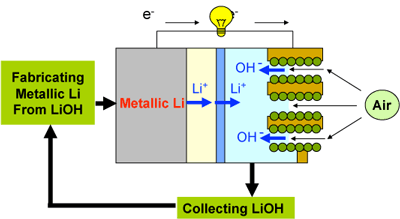 |
|
Fig. 4 Concept of a metallic lithium fuel cell in which lithium is used cyclically
|
The lithium-air battery newly developed by AIST needs further technical improvement toward practical use. Generally, there are two directions in this new lithium-air battery research, one is for rechargeable lithium air battery and the other is for lithium fuel cell.