Hiromichi Kataura (Leader) and Takeshi Tanaka (Research Scientist), the Self-Assembled Nano-Electronics Group, the Nanotechnology Research Institute (Director: Nobutsugu Minami) of the National Institute of Advanced Industrial Science and Technology (AIST) (President: Hiroyuki Yoshikawa) have developed an easy-to-use method to separate metallic and semiconducting single-wall carbon nanotubes (SWCNTs) using agarose gel.
Synthesis of SWCNTs usually results in a 1:2 mixture of metallic and semiconducting ingredients. Further separation of the ingredients is very important for electrical applications, but has been difficult so far.
AIST developed a separation method for metallic and semiconducting SWCNTs with a high yield by means of electrophoresis using agarose gel, as published in February 2008, and has since developed a much more simplified method as described below.
The new method simply freezes, thaws and squeezes SWCNTs-containing agarose gel (Fig. 1). The process is intrinsically simple, can be low-cost method through automation, and is easy to be scaled up. The process will then lead to mass production of metallic and semiconducting SWCNTs.
Part of the results of this study will be published in the U. S. scientific journal Nano Letters on March 11, 2009.
 |
|
Fig. 1 Separation of metallic and semiconducting SWCNTs by a freeze, thaw and squeeze method with SWCNTs-containing agarose gel. A solution containing metallic SWCNTs and a gel containing semiconducting SWCNTs are simultaneously obtained by squeezing the SWCNTs-containing gel after freeze and thaw processes. |
SWCNTs are classified into metallic and semiconducting types, depending on how the carbon atoms are arranged. Synthesis of SWCNT results in a mixture of the two types. Assuming that the two materials can be separated with high purity, we have many potential applications. Metallic SWCNTs could be used as transparent electrodes in liquid crystal displays or solar cells substituting transparent conductive materials using rare metals. Semiconducting SWCNTs could be used as transparent and flexible transistors. Using metallic SWCNTs in wiring and semiconducting SWCNTs in transistors, high-integration high-speed SWCNT computers can be realized in the future.
As no method is as yet available for independently synthesizing the two materials, which have different electric properties, there have been many attempts to separate each of the materials from their mixture. However, due to problems of low yield, purity, and cost, none of the proposed methods has been brought to mass production. Therefore, a low-cost separation method that can be brought into mass production has been sought.
AIST found that electrophoresis using SWCNTs-containing agarose gel can efficiently separate metallic and semiconducting SWCNTs with a high yield. (AIST press release, February 26, 2008) This method was expanded upon, and an easier, more efficient method has been devised.
Part of the research is supported by Industrial Technology Research Grant Program in 2008 from New Energy and Industrial Technology Development Organization (NEDO) of Japan, and by Core Research for Evolutional Science and Technology (CREST), Japan Science and Technology Agency.
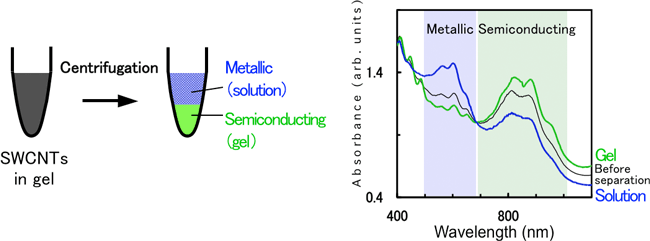
To clarify the mechanism of separation of metallic and semiconducting SWCNTs using agarose gel electrophoresis and further to develop a simpler method, separation methods that do not use an electric field are pursued. By applying a centrifugation to SWCNT-containing gel, the gel is condensed and a solution in the gel is squeezed out (gel centrifugation method, Fig. 2): this solution contains metallic SWCNTs, and the remaining gel debris contains semiconducting SWCNTs. Clearly, electric field for the electrophoresis is not necessary in the separation using agarose gel. A yield of almost 100%, similarly to gel electrophoresis, is obtained.
 |
|
Fig. 2 Separation of metallic and semiconducting SWCNTs by centrifugation method. Right figure shows optical absorption spectra before and after separation. |
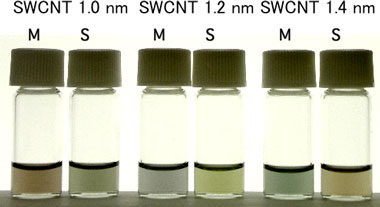
The freeze, thaw and squeeze method is applied to separate the solid and the liquid phase of the gel. Note that this method has long been applied to the production of freeze-dried tofu (bean curd), "Kouya-dofu", in order to remove water where the gel structure of tofu varies by freezing and thawing. When the SWCNT-containing gel is squeezed without freezing and thawing processes, the gel simply breaks. After freezing and thawing processes, however, the gel is separated into a solution containing metallic SWCNTs and a gel debris containing semiconducting SWCNTs just by squeezing the gel with the fingers as shown in Fig. 1. This method does not require any sophisticated apparatus--a domestic freezer would suffice. The semiconducting SWCNTs in the squeezed gel can be easily recovered by heating and melting the gel and mild centrifugation. This method is also capable for the separation of SWCNTs of different diameters (Fig. 3).
 |
|
Fig. 3 Separation of metallic and semiconducting SWCNTs with different diameters by the freeze, thaw and squeeze method. SWCNT solutions have different colors because the color depends not only on the metallic/semiconducting material type, but also on their diameter. M, metallic SWCNT solution; S, semiconducting SWCNT solution. |
It is very likely that the separation is achieved because the semiconducting SWCNTs are selectively adsorbed to the agarose gel.
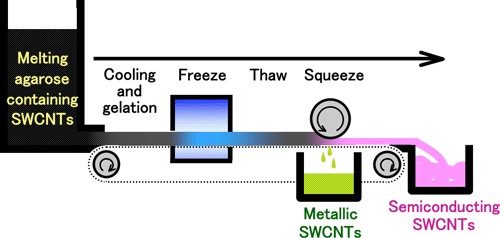
The method is so simple that we can easily proceed to the automation and scaling up of the process. For example, by using an automatic continuous equipment as shown in Fig. 4, kilograms of SWCNTs will be separated in a day.
 |
|
Fig. 4 Schematic of equipment for a continuous large-scale separation of metallic and semiconducting SWCNTs |
Larger-volume separation of metallic and semiconducting SWCNTs was attempted using the gel centrifugation method, and about 10mg of SWCNTs could be separated using a 500 ml plastic centrifugal bottle (Fig. 5). This amount is about a thousand times larger than that obtainable by the agarose gel electrophoresis.
 |
|
Fig. 5 SWCNT solutions separated by the gel centrifugation method using a 500 ml bottle (both ends). The two bottles in the middle contain diluted solutions to show the colors. |
The research for larger scale and lower cost production of metallic and semiconducting SWCNTs shall be pursued through cooperation with companies. In addition, applications of the two types of SWCNTs will be exploited.