- Expected to be used as a visible-light-driven photocatalyst for indoor applications -
Masahiro Miyauchi (Senior Research Scientist) and Zhao Zhigang (Post-Doctoral Research Scientist), the Nano-Structured Material Group, the Nanotechnology Research Institute (Director: Nobutsugu Minami) of the National Institute of Advanced Industrial Science and Technology (AIST) (President: Hiroyuki Yoshikawa) have successfully synthesized WO3 nanotubes by a simple hydrothermal method. These nanotubes are composed of aggregates of crystallites and have a nanoporous structure with fine, nanometer-scale pores on their walls. This structure provides the nanotubes with a large specific surface area, enabling high photocatalytic activity.
When used to decompose gas-phase acetaldehyde, these nanotubes with platinum as a promoter exhibit visible-light-induced photocatalytic activities eight times higher than that of conventional N-doped TiO2, and three times higher than that of conventional particulate WO3 with a platinum promoter.
Since the hydrothermal synthesis method can produce large quantities at low cost, it is expected that this novel catalyst will be used commercially in products such as "safe and healthy interior construction materials" that can decompose harmful volatile organic compounds (VOCs) to purify indoor environments, where little ultraviolet (UV) radiation exists.
The results of this research have been published in Angewandte Chemie–International Edition, a German academic journal published by Wiley-VCH.
 |
|
Images of tungsten oxide nanotubes observed usinga scanning electron microscope.
|
A photocatalyst, when exposed to light, decomposes harmful substances or exhibits antibacterial properties. Photocatalysts can also be coated onto the outer walls to make it difficult for dirt to attach to them, thereby producing "self-cleaning" surfaces. Titanium oxide (TiO2) is known as a typical photocatalyst, however it functions only when exposed to UV light. It is impractical in indoor environments, where little UV light exists. But the demand for photocatalysts that can work under indoor visible light is increasing. The photocatalysts can decompose harmful indoor VOCs, and can be a countermeasure against the sick house syndrome, for example. Although some visible light responsive photocatalysts such as N-doped TiO2 have been reported recently, none achieve practical levels of performance. However, recent research efforts have resulted in the discovery that nanoparticles of WO3, a simple oxide, together with promoters such as platinum or palladium particles or copper compounds embedded on their surfaces, exhibit high levels of activity when exposed to visible light. However, since commercially available WO3 particles are large in size and, therefore, low in specific surface area, they are not necessarily appropriate as a photocatalyst base material. Moreover, only few cases of WO3 nanoparticle synthesis have been reported, and the development of nanostructure-controlled WO3 nanoparticles is necessary for WO3-based photocatalysts with improved activities.
The Energy Technology Research Institute of AIST previously discovered that the activity of WO3-based photocatalysts can be greatly improved by simply mixing them with promoter particles of palladium or copper compounds (press release on July 9, 2008). At the same time, the Nanotechnology Research Institute of AIST focused its attention on the base WO3 particles themselves, and has been making efforts aimed at creating a high-activity photocatalyst by controlling the nanostructure.
This development is the fruition of research conducted in collaboration with the University of Tokyo as part of the "Project to Create Photocatalyst Industry for Recycling-oriented Society" initiated by the New Energy and Industrial Technology Development Organization (NEDO).
Figure 1 shows scanning electron micrographs of the synthesized WO3 nanotubes. Each nanotube is composed of aggregates of crystallites, each 100 nm in size or smaller, and has a nanoporous structure with many fine pores, several tens of nanometers in size, on the tube wall, producing a high specific surface area. The nanotubes have outer diameters of 300–1000 nm and lengths of 2–20 µm. They can be synthesized in high yield by simply heating the starting materials and the solvent in a sealed container. In this study, a high-yield synthesis process was developed by discovering that the introduction of urea to the hydrothermal reaction solution enabled the formation of nanotubes. Since an expensive templating agent is not necessary in the hydrothermal method used, a low-cost, large scale manufacturing process can be achieved.
 |
|
Figure 1. Images for tungsten oxide nanotubes by a scanning electron microscope.
|
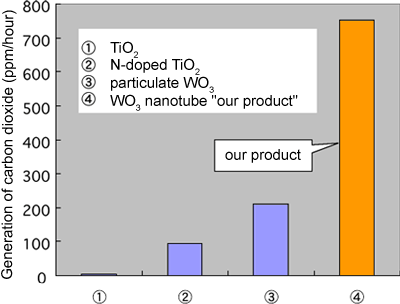
A gas-phase acetaldehyde decomposition test was conducted with the nanotubes obtained from this process, with fine particles of platinum applied to the surface of the nanotubes as promoters. Irradiation with visible light at wavelengths of 400 nm or longer initiated a reduction in the concentration of acetaldehyde and the simultaneous generation of carbon dioxide (CO2) as a decomposition product, showing the visible-light-induced photocatalytic activity. Figure 2 shows the rate of CO2 generation induced by visible light. The newly developed WO3 nanotubes (Fig. 2-4) showed approximately eight times higher activity than conventional N-doped TiO2 (Fig. 2-2). When compared with a platinum-supported commercial particulate WO3 (Fig. 2-3), the platinum-supported WO3 nanotubes showed more than three times higher visible-light-induced catalytic activity.
It was also confirmed that the newly developed WO3 nanotubes can completely decompose acetaldehyde to CO2 in the presence of visible light.
 |
|
Figure 2. Photocatalytic activity for decomposing acetaldehyde under visible light irradiation.
|
The high visible-light-induced activity of WO3 nanotubes is mainly due to their nanoporous structure, which provides them with a large specific surface area. In the future, we intend to further improve the activity by selectively introducing a promoter onto either the inner or outer walls of the nanotubes. We also intend to develop a process to manufacture nanotube thin-films for possible applications as coating materials.