- Complete oxidative decomposition of various volatile organic compounds under visible light -
Hideki Sugihara (Leader), Kazuhiro Sayama (Senior Research Scientist), Takeo Arai (Post-Doctoral Research Scientist) et al. of the Solar Light Energy Conversion Group, the Energy Technology Research Institute (Director: Yasuo Hasegawa) of the National Institute of Advanced Industrial Science and Technology (AIST) (President: Hiroyuki Yoshikawa) have developed a tungsten oxide (WO3) visible light responsive photocatalyst that can be activated to a high enough level to enable the complete oxidative decomposition of various volatile organic compounds (VOCs) under visible light illumination including fluorescent lighting at indoor or in vehicles, where little ultraviolet (UV) light exists.
Complete oxidative decomposition means completely oxidizing an organic substance by decomposing the substance into carbon dioxide (CO2) and water, and detoxifying it. We have succeeded in complete oxidative decomposition of VOCs including formaldehyde, acetaldehyde, formic acid, acetic acid, and aromatic compounds such as toluene, which is known as a persistent substance.
This photocatalyst has been realized by adding newly developed high-performance promoters, namely metallic palladium (Pd) and copper (Cu) compounds, to a WO3 semiconductor photocatalyst. Activity improves dramatically simply by mixing promoter particles into the WO3 powder. In acetaldehyde decomposition under visible light illumination, the Pd added WO3 photocatalyst showed an oxidative decomposition activity more than seven times that of a typical photocatalyst, titanium oxide (TiO2).
Various applications of the new photocatalyst are expected at indoor locations or in vehicles, where little UV light exists. The applications include the decomposition of organic solvents and sick house syndrome causative agents emitted from paints, adhesives, and building materials, the decomposition of malodorous substances, as well as in air cleaners.
 |
|
Photo : WO3 photocatalyst coated on glass plate
|
TiO2 based photocatalysts are used in a number of fields. They are used in a wide range of applications including air cleaners, air-conditioners, blinds, and wallpapers that exploit their harmful substance decomposition function, external wall materials, and window glass that have an antifouling function, antifogging side mirrors on vehicles, antibacterial tiles used in hospitals, and external wall-cooling technology that takes advantage of their superhydrophilic property. TiO2, however, has the drawback of being able to absorb and use UV light only.
UV light accounts for only 3% (energy equivalent) of sunlight, and fluorescent and incandescent lamps emit UV light only in minute amounts. The window glass (security glass and eco-friendly glass) used in the latest houses, and the windshields of automobiles also block almost all UV light. Therefore, there has been a need for the development of a new photocatalyst that works efficiently under visible light in indoor and in-vehicle environments where there is little UV light, to enable the decomposition and removal of organic substances that cause sick house syndrome or chemical sensitivity syndrome, of various VOCs, and of malodorous substances.
At AIST, we have carried out R&D of a visible light responsive photocatalyst that is used in the photolysis of water to generate hydrogen. We were the first in the world to develop a photocatalyst system that uses visible light to completely decompose water into hydrogen and oxygen (press release: December 6, 2001). In the same way that this catalyst system uses a platinum-loaded WO3 semiconductor photocatalyst, a WO3 semiconductor has been used for photolysis of water, but in few cases has WO3 been used to decompose harmful substances. This is because only a little activity can be obtained when WO3 alone is used, resulting in incomplete oxidative composition. However, WO3 has many advantages, such as its light absorption property - it can absorb a wavelength of up to 460 nm. We pressed ahead with development confident that practical complete oxidative decomposition could be achieved by exploiting this advantage.
This research was carried out in collaboration with the University of Tokyo as part of the "Project to Create Photocatalyst Industry for Recycling-oriented Society" (started in FY 2007) supported by the New Energy and Industrial Technology Development Organization (NEDO).
An analysis of the complete oxidative activity of photocatalysts comprising WO3 powder to which had been added an array of promoters showed that only platinum (Pt), Pd, and Cu compounds improved the oxidative decomposition of organic substances. As Pt is very expensive, the use of Pd and Cu compounds promotors, which are less expensive, were examined aiming at commercialization. Figure 1 shows the powder of WO3 photocatalysts we developed in this research, which was produced simply by mixing in a mortar WO3 powder with minute particles of promoter, Pd or copper oxide (CuO).

(a) Pd-WO3 photocatalyst powder
|

(b) CuO-WO3 photocatalyst powder
|
|
Figure 1 : photocatalyst powder
|
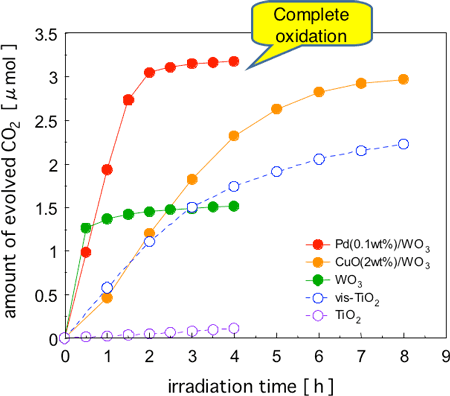
Figure 2 shows the changes in the amount of CO2 generated from the complete oxidative decomposition of acetaldehyde when each photocatalyst was irradiated with visible light. Acetaldehyde is a typical VOC used for comparison in photocatalyst activity. TiO2 (typical commercial product) showed hardly any activity when exposed to visible light only. As for the other catalysts, although gas phase acetaldehyde disappeared through irradiation by visible light, it was assumed that complete oxidation was difficult when using WO3 without a promoter or visible light responsive TiO2 (vis-TiO2) judging from the changes in the amount of CO2 generated. This is because oxidation stops midway when acetaldehyde has become acetic acid, formic acid, or formaldehyde, those are intermediates in the oxidation process, and further decomposition does not progress easily. Accumulation of acetic acid, formic acid, and formaldehyde on WO3 was also observed. If intermediates build up, this depresses activity and may worsen contamination. The performance of a catalyst should be evaluated not just by measuring how the organic substance to be decomposed has been reduced, but by evaluating whether complete oxidative decomposition (that is to say, complete detoxification) has been achieved, based on the increase in the amount of CO2 generated.
The activity of the TiO2 based photocatalyst dropped drastically during the latter part of the reaction, which may be attributed to inhibited working of the catalyst due to the accumulation of reaction intermediates. On the other hand, the Pd-loaded WO3 photocatalyst (Pd-WO3) maintained a high activity, and all acetaldehyde was completely oxidized and decomposed quickly. When compared at the midpoint of the reaction process, the activity (rate of CO2 generation) of Pd-WO3 was approximately seven times higher than that of vis-TiO2. The difference in the level of activity between the two catalysts became larger during the latter part of the reaction process. When CuO, which is even cheaper than Pd, was added as a promoter to WO3 (CuO-WO3), the oxidation activity was lower than that of Pd-WO3, however, the activity was approximately three times higher than that of vis-TiO2, judging from the rate of CO2 generation at the midpoint of the reaction.
 |
|
Figure 2 : Time courses of CO2 formation during photodegradation of acetaldehyde (ca. 1.6 µmol) over various catalysts under visible-light irradiation (λ > 400 nm).
|
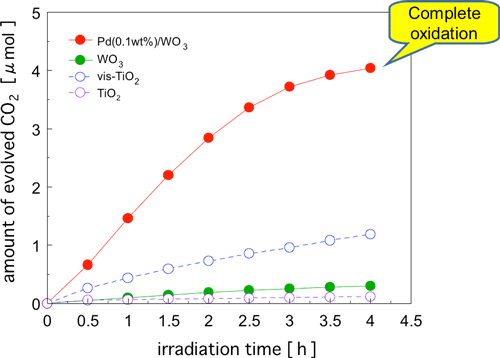
Next, we compared the different photocatalysts to see how well they were able to oxidatively decompose acetic acid, which is one of the typical reaction intermediates (Fig. 3). As in acetaldehyde decomposition, the Pd-WO3 photocatalyst showed a much higher activity than vis-TiO2, and complete oxidative decomposition was achieved in a short time. As for formaldehyde, which is another reaction intermediate and a causative agent of sick house syndrome, nearly complete oxidative decomposition was achieved by the use of Pd-WO3 and CuO-WO3 photocatalysts.
 |
|
Figure 3 : Time courses of CO2 formation during photodegradation of acetic acid (ca. 2 µmol) over various catalysts under visible-light irradiation (λ > 400 nm).
|
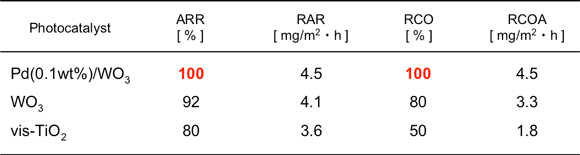
We also evaluated the activity of different photocatalysts in a flow reactor experiment using low levels of gas concentration simulating the conditions of actual use (Table 1). In this experiment, a fluorescent lamp with an acrylic window was used as the light source on the assumption that the hoods of lighting equipment are coated with a photocatalytic membrane. When using WO3 without a promoter and vis-TiO2, the rate of oxidation was less than 100%, and large amount of intermediates accumulated or diffused. The rate of acetaldehyde removal was also less than 100%, and much of the acetaldehyde passed through the flow system without reacting on the catalyst. On the other hand, both the oxidation and removal rates were 100% when Pd-WO3 was used, indicating that all inflow acetaldehyde was completely oxidized to CO2. This means that Pd-WO3 showed higher performance than vis-TiO2 in the flow experiment as well. CuO-WO3 also showed oxidation and removal rates of nearly 100% in the flow system under fluorescent lighting with a low concentration level of 2 ppm.
|
Table 1 : Removal of acetaldehyde from flowing gas (5 ppm, 50 ml/min) over Pd(0.1 wt%)/WO3, WO3, and N-TiO2 under fluorescent-lamp irradiation (ca. 3000 lux, with acrylic window, Catalyst area: 59 cm2).
|
 |
|
ARR: The acetaldehyde removal ratio,
RAR: The rate of acetaldehyde removal,
RCO: The ratio of complete oxidation of acetaldehyde to CO2,
RCOA: The rate of complete oxidation of acetaldehyde
|
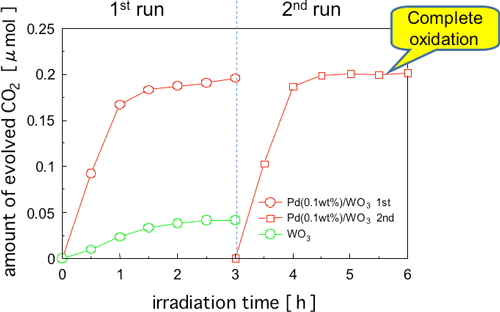
Figure 4 shows the results of oxidative decomposition of toluene, which is a highly persistent typical aromatic VOC, using Pd-WO3. Toluene is a VOC that is used as a diluent for paints and adhesives. The level of activity was remarkably low when WO3 without a promoter was used, and CO2 generation was decreased during the reaction due to the accumulation of reaction intermediates on the surface of the photocatalyst inhibiting the activity. On the other hand, the activity level was much higher with Pd-WO3, and it is assumed that complete oxidative decomposition was achieved judging from the amount of CO2 generated. It was also confirmed that there was little deterioration in the level of photocatalyst activity after repeated reactions.
 |
|
Figure 4 : Time courses of CO2 formation during photodegradation of toluene (ca. 0.03 µmol) over various catalysts under visible-light irradiation (λ > 400 nm). The catalysts were cleaned by means of a pre-photoreaction before measurement
|
As described above, a WO3 visible light responsive photocatalyst loaded with appropriate promoters enabled complete oxidative decomposition (complete detoxification) for many harmful organic substances at efficiency levels higher than with TiO2 based photocatalysts. WO3 is highly stable and composed of nontoxic elements. Moreover, the photocatalysts can be produced in large amounts using a simple mixing method. Therefore, it is a semiconductor photocatalyst with many properties that are advantageous in commercial production and use.
For these new photocatalysts to be commercialized, it is necessary to develop 1) a technology for producing the catalysts at lower costs, and 2) a technology for forming a membrane with practical strength without reducing the photocatalytic activity. As for 1), as the raw material cost of the newly developed photocatalysts are more expensive than that of TiO2, realization of value-added applications will be pursued at an initial stage. These applications include the use of the catalyst for the hoods of lighting equipment and high-performance air cleaners, as well as in automobiles. We will also look at the development of an optical confinement technology for efficient absorption of light and reduction of the amount of catalyst used. As Pd promoters are more expensive than copper compounds, we are examining ways to further reduce the amount of promoter required. Expected applications of Pd-WO3 include filters in high-performance air cleaners and the purification of paint factories, while possible applications of CuO-WO3, which is inexpensive and has potential antibacterial properties, are tiles for use in hospitals, hoods of household lighting equipment, wallpaper, and window blinds. As for 2), the Solar Light Energy Conversion Group will take advantage of its thin-film formation technology to further research encompassing an improvement in long-term reliability.