Associate Professor Yutaka Maniwa's group with the Graduate School of Science and Engineering of Tokyo Metropolitan University (President: Junichi Nishizawa) and Hiromichi Kataura, chief of the Self-Assembled Nano-Electronics Group at the Nanotechnology Research Institute of the National Institute of Advanced Industrial Science and Technology (President: Hiroyuki Yoshikawa) (hereinafter referred to as AIST) jointly demonstrated the adsorption of water molecules into a Single-Walled Carbon Nanotube (SWCNT) under different gas ambiences and discovered the "exchange transition" phenomenon involving ambient gas and water molecules.
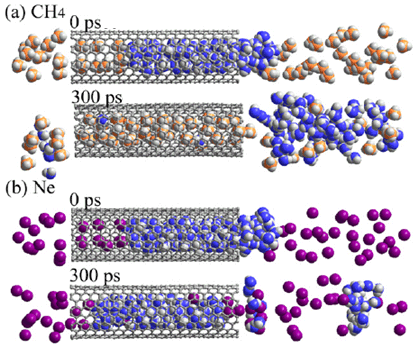
The "exchange transition"-characterized as an exchange between molecules of water inside the SWCNT and ambient gas molecules -was verified for seven types of ambient gas: argon, krypton, oxygen, nitrogen, methane, ethane, and carbon dioxide. The conditions for occurrence of exchange transition depend on the type of gas. For example, with methane at one atmosphere and -30°C or below, water molecules are expelled from the SWCNT and replaced by methane molecules which penetrate into the SWCNT. In contrast, when helium, hydrogen, or neon was used, water molecules remained stable inside the SWCNT at temperatures of -170°C or below (Figure 1). Using this phenomenon, the water-filled SWCNT can be used as a molecule-selective nanovalve.
In addition, the sudden change in electrical resistance of the SWCNT film due to the exchange transition can be used to create a new gas sensor that permits the selection of gases without any special chemical treatment, coating, or the like. Tokyo Metropolitan University and AIST plan to put the gas sensor and the molecule-selective nanovalve to practical use and invite the participation of companies possessing related technologies.
The results of the present research were published in the on-line version of the scientific journal Nature Materials under the title "Water-filled single-wall carbon nanotubes as molecular nanovalves" on January 21, 2007 (GMT). Part of it has received support from the Japan Science and Technology Agency/Core Research for Evolutional Science and Technology (CREST).
 |
|
Figure 1. Exchange transition for CH4 and Ne. (a), (b); molecular dynamics simulations for the coexistent systems of water and CH4 (a) and Ne (b) molecules. Initially the water clusters are located inside SWCNT. After 300 ps, CH4 molecules enter the SWCNT, although Ne molecules cannot enter. |
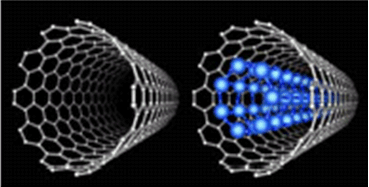
The behavior of water and gas molecules in nanospace is an important issue in such areas as nanotechnology and energy storage but has not been fully explained. It was previously known that water can be easily adsorbed in a normal environment into a single-walled carbon nanotube (SWCNT) containing a cylindrical cavity about 1 nm in diameter (Figure 2) in spite of the hydrophobic nature of the SWCNT wall, but the behavior with a coexisting gas was totally unknown.
 |
|
Figure 2. Schematic illustration of SWCNTs and SWCNTs with ice NTs. |
The phenomenon of water adsorption into hydrophobic SWCNT was suggested in 1999 by Dr. Maniwa with the Graduate School of Science of Tokyo Metropolitan University (currently the School of Science and Engineering of Tokyo Metropolitan University) and Dr. Kataura (currently with AIST), and later experimentally confirmed in 2002. Moreover, it was demonstrated that the ring-shaped ice formation (ice nanotube or ice-NT) theoretically predicted by Drs. Koga and Tanaka (currently with the School of Science of Okayama University) was actually observed inside the SWCNT. The dependency of the formation of ice-NT and its structure on the SWCNT diameter was further investigated and it became clear that the melting point increases with smaller diameters, and that thin nanotubes allow for the formation of ice-NT at room temperature. Unlike previous experiments which were carried out in vapor ambiences, this time we examined in detail the adsorption of water into the SWCNT when coexisting with several types of gases.
Electrical resistance measurements, NMR tests, X-ray diffractometer (XRD) tests and computer simulation were carried out using a high-purity SWCNT material in whose preparation the diameter was set to 1.35nm by means of control based on the laser ablation method. XRD tests were carried out at Photon Factory BL1B of the High Energy Accelerator Research Organization, an inter-university research institute corporation.
Ten types of gases (hydrogen, helium, neon, argon, krypton, oxygen, nitrogen, methane, ethane, and carbon dioxide) were investigated under one atmosphere at a temperature range from room temperature down to -180°C. As a result, we discovered that at low temperature or high pressure, water molecules inside the SWCNT exchanged with molecules from the gas ambience ("exchange transition").
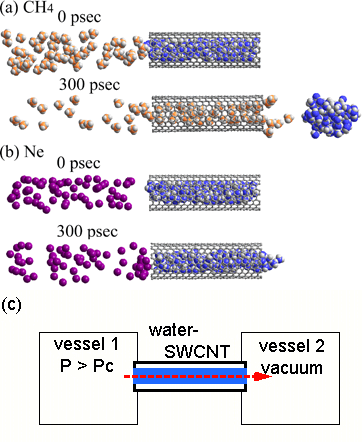
The temperature at which exchange transition occurs depends heavily on the type of gas and pressure. For instance, using methane under one atmosphere, water molecules inside the SWCNT were expelled and replaced by methane which penetrates into the SWCNT at temperatures below approximately -30°C. On the other hand, using helium, hydrogen, or neon, water molecules remained stable inside the SWCNT at temperatures as low as -170°C (Figure 1). The clear dependency on the type of gas as illustrated in Figure 1 can be explained as a phenomenon peculiar to nanospaces. In addition, computer simulations showed that this phenomenon makes it possible to use water-filled SWCNT as a molecule-selective nanovalve (Figure 3).
 |
|
Figure 3. Valve function of water-SWCNTs. (a), (b); A demonstration of the water-SWCNT valve afforded by MD simulations for CH4 (a) and Ne (b). Initially, the water clusters are positioned inside the SWCNT to prevent gas molecules from passing through the SWCNT. After 300 ps at 300 K, the CH4 molecules force the water out, allowing CH4 to pass through to the right-hand side of the SWCNT (the right vessel 2 in (c)). Ne molecules on the other hand, are not able to pass through the blocked SWCNT. |
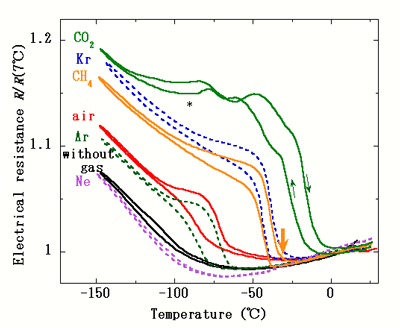
Moreover, measurements of the dependency of electrical resistance on temperature indicated sudden changes in electrical resistance of the SWCNT film due to exchange transition (Figure 4). These changes can be applied in new types of gas sensors that can select gases without any special chemical treatment or coating.
 |
|
Figure 4. Temperature dependence of electrical resistance of water-SWCNT films in various gases at 1 atm, normalized at 7°C. A thick arrow indicates the exchange transition temperature for CH4. |
Name of the journal: Nature Materials,
Title: Water-filled single-wall carbon nanotubes as molecular nanovalves
Co-authors: Yutaka MANIWA, Kazuyuki MATSUDA, Haruka KYAKUNO, Syunsuke OGASAWARA, Toshihide HIBI, Hiroaki KADOWAKI, Shinzo SUZUKI, Yohji ACHIBA and Hiromichi KATAURA