Researchers) LIN Yung-Chang, Senior Researcher, Electron microscopy group, Nanomaterials Research Institute, SUENAGA Kazutomo, Institute of Scientific and Industrial Research, Osaka University, MATSUMOTO Rika, Tokyo Polytechnic University, AGO Hiroki, Global Innovation Center, Kyushu University, CHIU Po-Wen, Department of Electrical Engineering, National Tsing Hua University
- Discovery of alkali metal bilayer structure in graphene interlayer
- Alkali metals between graphene layers are tightly packed due to the inherent extensibility of the graphite surface between layers.
- Expected to increase capacity of rechargeable batteries for electric vehicles and telecommunications equipment with two to few-layer graphene electrodes
Alkali metals form a hexagonal close-packed bilayer when inserted into bilayer graphene
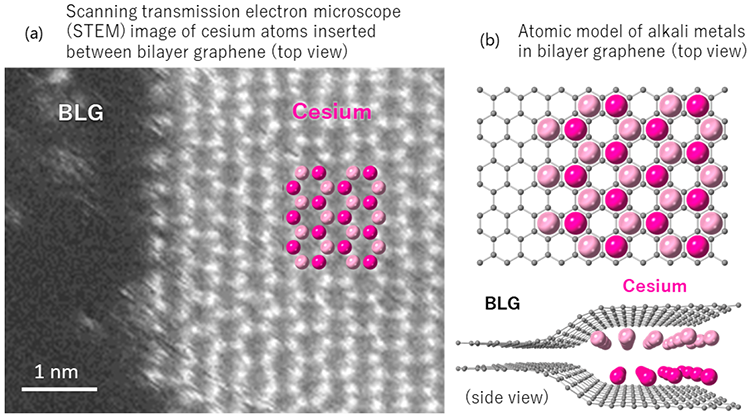
Researchers at AIST, in collaboration with Osaka University, Tokyo Polytechnic University, Kyushu University, and National Tsing Hua University, have developed a technique to insert alkali metals into the interlayers of graphene. Graphene is a single layer of carbon atoms arranged in a hexagonal lattice. They have succeeded in directly observing the atomic arrangement of the inserted alkali metal atoms which is a hexagonal close packed bilayer structure.
The performance of rechargeable batteries is a key factor influencing the driving distance of electric vehicles and the usage time of smartphones. Improving the performance of these electronic devices is possible if rechargeable batteries can accumulate greater electrical capacities. Graphite, the electrode material used in batteries, is composed of multilayers of graphene, with alkali metals placed between the layers to facilitate the flow of electrons during charging and discharging. Achieving a high density of alkali metals storage between graphene layers could increase the electric capacity.
For the past hundred years, it has been widely recognized through X-ray and electron diffraction measurements that graphene interlayers can only accommodate a single layer of alkali metal. Each layer being fully filled by single layer alkali metal atoms is considered the theoretical charging limit. However, there have been no reports of studies directly observing the atomic arrangement of interlayer alkali metals and verify whether graphene layers can only accommodate a single layer of alkali metal atoms or whether other techniques can achieve higher density or multiple layers of alkali metals.
We have developed a technique to insert dense alkali metals between graphene layers. Utilizing a high-performance low-voltage (60 kV) electron microscope, we have successfully observed the arrang11ement structure of alkali metal atoms between the graphene layers. The alkali metals are found densely packed in a two-layer structure in both bilayer graphene and in the surface layer graphite due to the flexible extension ability of their interlayer spacing. This allows approximately twice as many alkali metals to be inserted. If graphene with two layers of alkali metal insertion can be stacked, it is expected to serve as an electrode material enhancing the capacity of alkaline ion secondary batteries.
The development of rechargeable batteries for use in electric vehicles and information equipment is a key strategic focus for Japan, aiming to achieve a decarbonized society and an advanced information network connecting people, goods, and services. Elemental technologies essential for enhancing the performance of rechargeable batteries include the imperative task of reducing battery weight and increase battery capacity. Graphite, composed of carbon layers, serves as a lightweight and robust electrode materials. During the charging and discharging process, electrons are transferred to and from alkali metal ions such as lithium. To increase the capacity of rechargeable batteries, it becomes crucial to incorporate more alkali metal ions into the electrodes. However, traditional measurement and evaluation techniques have posed challenges in observing the electrode structure at the atomic level. Additionally, design guidelines for effectively inserting alkali metal ions into graphene layers have been severely limited.