Update(MM/DD/YYYY):10/02/2019
Discovery That a Blue Pigment Is a High-Performance Ammonia Adsorbent
– Expected to be used for malodor removal, PM 2.5 countermeasures, and hydrogen purification for fuel cells –
Points
-
Discovered that the ammonia adsorption capacity of a blue pigment, Prussian blue, is superior to conventional ammonia adsorbents
-
Controlled the structure of Prussian blue at an atomic level with element substitution and defect introduction to increase ammonia adsorption capacity
-
Adsorbed ammonia at such odorless, low concentrations, being expected to be utilized for removal of malodor and a PM 2.5 causative substance
Summary
Akira Takahashi (Researcher), Toru Kawamoto (Leader) and others, the Nanoparticle Functional Design Group, the Nanomaterials Research Institute (Director: Takeshi Sasaki), the National Institute of Advanced Industrial Science and Technology (AIST; President: Ryoji Chubachi), in collaboration with Shin-ichi Ohkoshi (Professor), the Department of Chemistry, the School of Science, the University of Tokyo (President: Makoto Gonokami), have discovered that the blue pigment Prussian blue has a higher adsorption capacity than common ammonia adsorbents, and controlled the structure of Prussian blue to synthesize Prussian blue analogues with higher ammonia adsorption capacity.
Prussian blue is a pigment used since early times. In the present study, the researchers found that Prussian blue adsorbs more ammonia than common adsorbents such as zeolite and activated carbon. In analogues with the metal ions included in Prussian blue replaced by other metal ions and more defects, the amount of absorbed ammonia increased. Furthermore, while common ammonia adsorbents have low adsorption capacity for low concentration ammonia, Prussian blue was able to adsorb low concentration ammonia in the air at “odorless levels.” It was also confirmed that the Prussian blue analogues can release ammonia once adsorbed, making them reusable.
This technology is expected to be used as an ammonia odor countermeasure in care homes, a technology for suppression of PM 2.5 generation, and a technology to remove ammonia in hydrogen fuel.
Details of this technology will be published in an American chemistry journal, Journal of the American Chemical Society, and were published online (Just Accepted Manuscript) on May 5, 2016 in advance.
 |
|
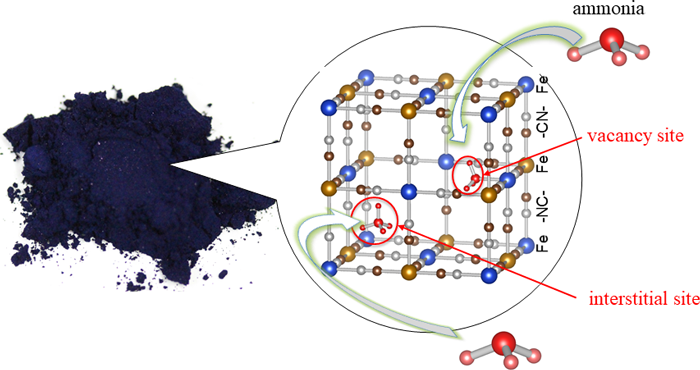
(Figure): Prussian blue (left) and the Prussian blue crystal structure that adsorbs ammonia molecules (right) |
Social Background of Research
Ammonia is the most produced chemical substance in the world, with its primary uses as a raw material for chemical products such as fertilizers and fibers. Yet, ammonia is also a malodorous substance, and urine, for example, decomposes to ammonia and causes malodor. Also, ammonia in the atmosphere is a causative substance of the fine particulate matter PM 2.5, believed to mainly originate from ammonia dissipating from agriculture and the livestock industry. Therefore, a technology to remove dilute ammonia contained in the atmosphere is required. In addition, if ammonia is contained in hydrogen supplied to a fuel cell, it has an adverse effect on power generation capacity of the fuel cell, so international standards on hydrogen for fuel cell vehicles require ammonia concentration of less than 0.1 ppm. Particularly in Japan, the government is advancing the development of technology to make hydrogen from ammonia, so a technology to remove ammonia from hydrogen fuel is crucial.
Currently, activated carbon, zeolite, and ion-exchange resins are used as common ammonia adsorbents. However, these adsorbents have issues such as difficulty in reuse, low adsorption capacity for low concentration ammonia, and high prices. So, there has been demand for low-price and reusable ammonia adsorbents that demonstrate high adsorption capacity even for low concentration ammonia.
History of Research
Recently, porous coordination polymers composed of metal ions and small molecules, with fine spatial networks inside, have gained attention as new materials for gas adsorption and recovery. AIST has conducted research and development of harmful substance removal using porous coordination polymers. In particular, AIST has advanced development using porous coordination polymers, i.e. Prussian blue-type complexes, to adsorb radioactive cesium with high efficiency, and use them in a volume reduction technology for plant-based contamination.
In the present study, the researchers used the structure of Prussian blue and Prussian blue analogues to develop an ammonia gas removal technology, while also pursuing improvement of ammonia adsorption capacity by structural control at an atomic level.
Details of Research
Prussian blue is a blue pigment with an over 300 years history, and was used for painting by Vincent van Gogh, Katsushika Hokusai, and others. Prussian blue has a structure in which iron ions (Fe) and hexacyanoferrate ions ([Fe(CN)6]) are connected three dimensionally, with approximately 0.5 nanometer (nm) microscopic spaces (interstitial sites) that can capture ammonia (Fig 1 (a)). The structure of Prussian blue can be controlled at an atomic scale, for example replacing iron ions with other metal ions or making defects where the hexacyanoferrate ions ([Fe(CN)6]) are missing (Fig. 1 (b)). In the present study, the researchers focused on the fact that exposed metal ions in these defects (vacancy sites) easily form coordination bonds with molecules, and investigated whether insoluble Prussian blue with defects are capable of high density adsorption of ammonia or not. In order to increase vacancy sites as much as possible, a method was devised to increase the numbers of defects, while reducing the content of alkali metal ions that are likely to induce the interstitial sites. The researchers thereby created a cobalt-substituted Prussian blue analogue (Co[Co(CN)6]0.60, CoHCC) and a copper-substituted Prussian blue analogue (Cu[Fe(CN)6]0.50, CuHCF) and evaluated their ammonia adsorption capacity along with Prussian blue.
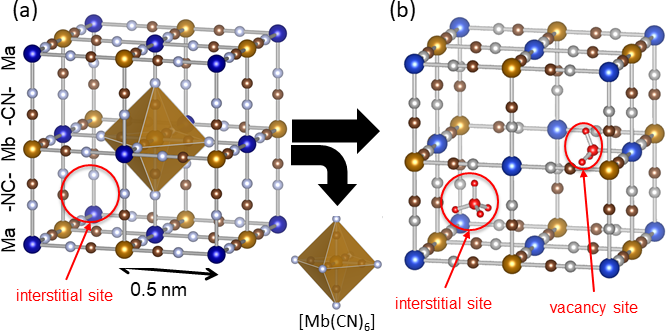 |
Figure 1: Structure of Prussian blue analogues
(a) Crystal structure and interstitial sites when there are no hexacyanometallte ion [Mb(CN)6] defects, and (b) interstitial sites and vacancy site when there are [Mb(CN)6] defects. When element Ma and Mb are both iron, it is Prussian blue. |
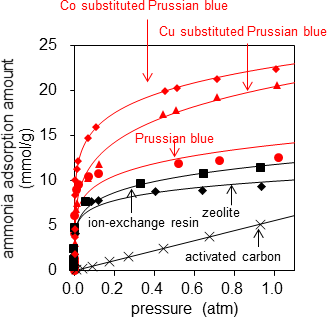
First, the researchers evaluated the adsorption amount in pure ammonia as the basic performance of adsorbents. Figure 2 shows the relationships between ammonia pressure and the adsorption amounts when Prussian blue, CoHCC, and CuHCF were placed in ammonia respectively. It also shows the data of the types with highest adsorption amount from a document evaluating and comparing various products for the common adsorbents such as ion-exchange resins, zeolite, and activated carbon1). The ammonia adsorption amount of Prussian blue was 12.4 mol (211 g)/kg at 1 atm, a higher value than common adsorbents. This corresponds to adsorption of 11 ammonia molecules per unit cell of Prussian blue with a volume of approximately 1 nm3. Furthermore, the analogues CoHCC and CuHCF showed high adsorption amounts of 21.9 mol (373 g)/kg and 20.6 mol (351 g)/kg respectively. CoHCC in particular had an ammonia adsorption amount of 16.2 molecules per unit cell, adsorbing 93 % of the estimated maximum adsorption amount of 17.6 molecules.
 |
|
Figure 2: Relationships between ammonia pressure and adsorption amounts at 25°C |
Next, the researchers placed a film of Prussian blue in a usual laboratory showing ammonia concentration of 0.015 ppm, and examined the adsorption behavior of dilute ammonia. As a result, the ammonia adsorption amount of the Prussian blue film increased with time, showing an adsorption amount of 0.3 mol (5.1 g)/kg (Fig. 3 (a)). This means that dilute concentration ammonia contained in the atmosphere was adsorbed and trapped in the fine space of Prussian blue that corresponds to 1 part in 700,000,000 by volume conversion. It is thought that Prussian blue can adsorb such dilute ammonia because adsorbed ammonia (NH3) reacts with water in Prussian blue to form an ammonium ion (NH4+), is stabilized, and is trapped inside Prussian blue without being rereleased into the air. The ammonia adsorption capacity of the ion-exchange resin (Amberlyst) and zeolite in a room, where ammonia of the same concentration is contained. Zeolite adsorbed almost no ammonia at all. The ion-exchange resin showed similar adsorption capacity to that of Prussian blue, but it is extremely expensive. These facts indicated the superiority of Prussian blue.
Furthermore, in order to check that ammonia is adsorbed by Prussian blue quickly enough, the researchers filled a thin tube with Prussian blue, and let air containing approximately 1 ppm of ammonia pass through at a speed so that the Prussian blue and air were in contact for 2 milliseconds only. As shown in Fig. 3 (b), after air with an ammonia concentration of 0.86 ppm passed through the tube, it decreased to 0.036 ppm, adsorbing and removing 96 % of ammonia. In addition, in the tests conducted in the same way, both CuHCF and CoHCC adsorbed and removed over 90 % of ammonia.
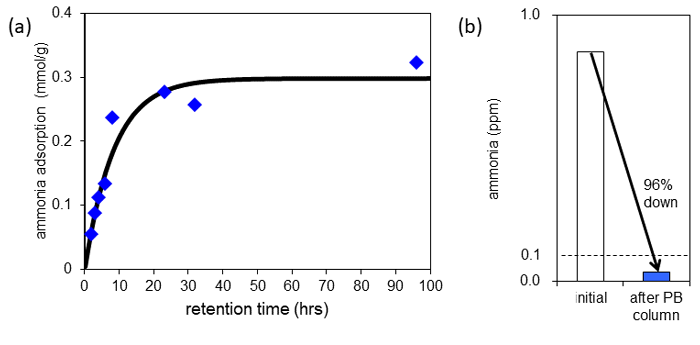 |
Figure 3: Prussian blue adsorption behavior of low concentration ammonia in the air
(a) Ammonia adsorption amount of Prussian blue film placed in the air. It increases along with time, reaching equilibrium after 24 hours. (b) The change in ammonia concentration before and after venting ammonia-containing air through a tube filled with Prussian blue powder. |
Finally, the researchers checked whether the newly fabricated analogues could be used repeatedly as adsorbents. As a result, in applications that removed dilute ammonia from the atmosphere, the adsorbed ammonia was desorbed by rinsing CuHCF with a dilute acid, and CuHCF was found to be reusable as an adsorbent. Also, in applications to store pure ammonia, CoHCC was capable of repeated use.
Future Plans
The Prussian blue analogues used in this study are similar to materials used as radioactive cesium adsorbents so far, and there are a variety of forming techniques for the radioactive cesium adsorbents, such as granules and non-woven fabrics supporting an absorbent. AIST will continue development so that Prussian blue and its analogues can be used as ammonia adsorbents, such as developing non-woven fabrics supporting Prussian blue to install them on ventilators in facilities which have potential for ammonia to dissipate, including in pig houses and compost buildings, and remove ammonia that can cause malodors and PM 2.5, and developing gas vent pipes coated with Prussian blue on their inner surface that can be installed in hydrogen stations to remove ammonia. In addition, AIST plans to search companies for joint research and technology transfer, and aims at practical use of ammonia removal and ammonia storage.
1) Helminen, J.; Helenius, J.; Paatero, E.; Turunen, I. Adsorption “Equilibria of Ammonia Gas on Inorganic and Organic Sorbents at 298.15 K.” J. Chem. Eng. Data 2001, 46 (2), 391–399