Using hepatitis B virus (HBV) reverse transcriptase (RT)-chimeric human immunodeficiency virus (HIV) RT, the researchers in AIST elucidated the mechanism of action of entecavir, a potent nucleoside analog drug for hepatitis B treatment, and the mechanism of drug resistance to entecavir, in collaboration with the National Center for Global Health and Medicine Research Institute.
 |
|
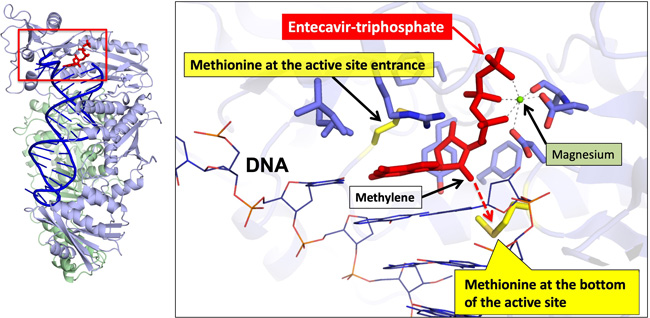
Structure of a modified reverse transcriptase of HIV bonded with entecavir |
For hepatitis B treatment, nucleoside analogs such as entecavir (ETV) have been used to block the action of RT, thereby inhibiting the infectivity and replication of HBV. As in the case of treatment of HIV infection, HBV variants resistant to such nucleoside RT inhibitors (NRTIs) emerge in NRTI-receiving individuals. New hepatitis B drugs that are effective against the drug-resistant HBVs and resist to the emergence of drug resistance are needed. However, HBV RT is unstable, making it highly difficult to solve its three-dimensional structure, hindering the development of new anti-HBV therapeutics.
HIV RT is fairly stable and the structure of its active site resembles that of HBV RT. The researchers generated a modified HIV RT (HBV RT-chimeric HIV RT) that binds to ETV and successfully analyzed its three-dimensional structure. They found that hydrophobic interactions of ETV are critical for its entry and binding to the active site of the chimeric RT. They also found that due to a hydrophobic interaction with the bottom of the active site, ETV bonds stably to the chimeric RT and inhibit its activity. In RT of an ETV-resistant HBV variant, the bottom of the active site turns into a small amino acid. The data explain how HBV acquires drug-resistance, i.e., ETV no longer stably bonds to HBV RT.
The researchers will further generate various modified HIV RT species that more closely resemble HBV RT, analyze their drug sensitivity, and the bonding mechanism of other nucleoside analog drugs, which should give insights into the design and development of new therapeutics to treat HBV infection.