Update(MM/DD/YYYY):10/05/2015
Visualization of Light Elements Such as Lithium at the Atomic Level
– Development of technique to directly “observe“ individual atoms of light elements –
Points
-
New method to visualize light elements such as lithium, of which observation by electron microscopes has been difficult
-
By confining light elements in carbon nanotubes and fullerene, reduce damage by an electron beam
-
Expectations for the analysis of lithium atoms in chemical reaction processes of secondary batteries
Summary
Kazutomo Suenaga (Prime Senior Researcher) of the Nanomaterials Research Institute (NMRI; Director: Tsuyoshi Sasaki), the National Institute of Advanced Industrial Science and Technology AIST; President: Ryouji Chubachi), and Ryosuke Senga (Researcher) of Electron Microscopy Group, NMRI, AIST, have succeeded in visualizing light elements including lithium at the precision level of individual atoms.
It has been considered difficult to observe lithium by electron microscopes. In this research, the researchers have succeeded in the visualization of individual lithium atoms for the first time, by confining lithium atoms in nano spaces and simultaneously implementing imaging by a low acceleration electron microscope and element analysis by electron energy loss spectroscopy (EELS). As in the case of lithium, the researchers were able to visualize individual atoms of chlorine, sodium, and fluorine, which have also been difficult to observe by electron microscopes.
This research has been carried out as part of the Strategic Creation Research Promotion Program of the Japan Science and Technology Agency (JST) and Grants-in-Aid for Scientific Research of the Japan Society for the Promotion of Science. Details of the research will be published online in Nature Communication on July 31, 2015 (Japan time).
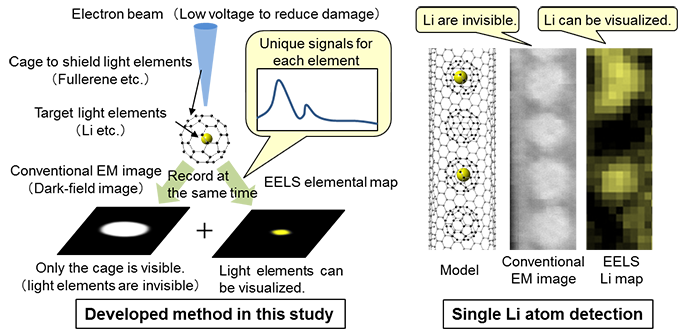 |
Schematic illustration of the developed method (left) and images of single-atom lithium actually taken (right)
EM: Electron microscope |
Social Background of Research
Since lithium is one of the elements that are used in a variety of industrial products, such as secondary batteries, and play important roles, it is essential to study the structure and behavior of lithium in detail in order to improve the performance of such products. Although there are various methods to investigate atoms, methods using electron microscope are ideal from the viewpoint that the state and behavior of atoms can be investigated directly. Usually, an electron microscopes forms images by detecting scattered electrons and so on, by radiating electrons to a sample and colliding with atoms in the sample. However, light elements such as lithium are very difficult to be observed by electron microscopes. This is due to the fact that the number of scattered electrons caused by collisions with lithium is extremely small in comparison with those of heavy elements and a clear image cannot be obtained.
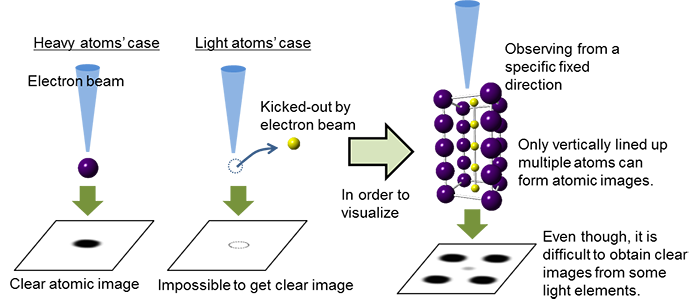
The fact that atoms of light elements are easily flicked by the electron beam, is also a major problem. Therefore, unless multiple atoms are lined up vertically in a crystal with sufficient thickness (an atom column), the light element will not be observed using a conventional electron microscope (Fig. 1). However, in case of a secondary battery where lithium moves between the substances during the chemical reactions, lithium atoms are not always lined up regularly. To examine such “atoms in an unknown state,” technology for the direct “observation” of individual atoms of light elements including lithium has been desired.
 |
|
Figure 1: Observation of light element by conventional electron microscope method |
History of Research
AIST has developed techniques to analyze elements at a precision level of a single atom (AIST press releases on July 6, 2009, January 12, 2010, December 16, 2010, and July 9, 2012). Also, AIST has developed a technique for position control of individual atoms by composing an atom chain consisting of two kinds of elements, etc. (AIST press release on September 16, 2014). In this research, by combining these techniques, the researchers have focused on the single atom analysis of lithium, which has been considered to be difficult.
The present research was supported by the JST Strategic Creation Research Promotion Program “Development of a low acceleration electron microscope to analyze the function of substance and life at atom level” (FY2012 - FY2016, Research Head: Kazutomo Suenaga) and Grants-in-Aid for Scientific Research: Conducted by young researchers (B) “Fundamental technology development for the assessment and application of atomic scale of a low dimensional material using nano base” (FY2014 - FY2016, Research Head: Ryosuke Senga) of the Japan Society for the Promotion of Science.
Details of Research
Combining a low acceleration electron microscope and EELS, the researchers have analyzed single atoms of lithium (Li), sodium (Na), fluorine (F), and chlorine (Cl).These elements are highly reactive, usually bond to other elements, and exist as compounds. However, when trying to observe the light elements in such compounds by an electron microscope, the light atom is flicked by the electron beam and the compound is easily destroyed. In this study, through the use of carbon nanotubes or fullerene as the shielding material in which the light element and the compound containing the light element are confined, damage by the electron beam has been reduced. Furthermore, in order to observe a single atom, the compound was thinned to the width of a single or double atom line. In Fig. 2, conventional electron microscope images (left) and the electron microscope images by the developed method (right) of atom chains containing various kinds of light elements confined in a carbon nanotube are shown. Comparing the structure models of each atom chain and conventional electron microscope images, although the heavy elements are clearly observed as bright spots, the images of the light atoms are not clear, and what kind of elements they are and even whether they exist cannot be ascertained. On the contrary, identifying images of each element and location of each element by using the developed method, and comparing the images with conventional electron microscope images, it is clearly confirmed that light element atoms exist between heavy atoms.
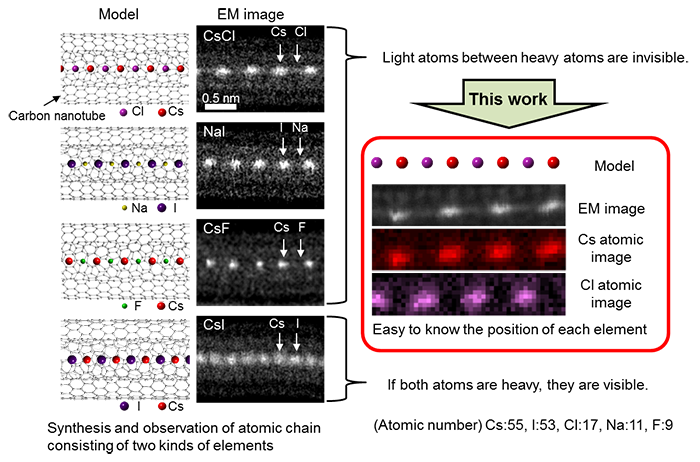 |
Figure 2: Visualization of light elements contained in atom chains
Cs: cesium I: iodine |
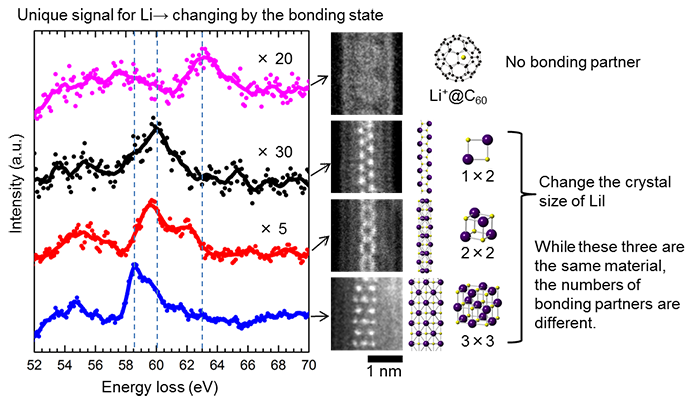
The developed method can be used not only for the identification of light element locations but also for understanding the chemical properties of each atom. For example, for lithium atoms in different states, by comparing the lithium signals obtained by EELS (Fig. 3), it can be understood that the signals are different according to the number of bonding atoms. In this way, from the signal form and peak position obtained from EELS, the chemical state (the information on how the atom bonds to which elements) can be understood. Until now, the process of presuming the lithium state from the result of analysis of different atoms which bond to the lithium atom has been mainly used. However, the bonding state of light elements such as lithium can be analyzed directly by the developed method. Therefore, there is a possibility that the lithium state in chemical reaction processes can be understood more precisely than when using conventional methods.
 |
|
Figure 3: Differences in chemical properties of lithium atom |
Future Plans
Although the researchers have used shielding materials, such as carbon nanotubes, in order to reduce the damage by the electron beam, they intend to focus on the development of measurement methods under conditions with less damage, aiming at the observation of light elements without any shielding material. This would realize the direct observation of light elements during chemical reaction processes.