- Using soft X-ray emission spectroscopy to reveal the behavior of electrons associated with charging and discharging -
Haoshen Zhou (Leader), Daisuke Asakura (Researcher), and Eiji Hosono (Senior Researcher) of the Energy Interface Technology Group, the Energy Technology Research Institute (Director: Haruhiko Obara), the National Institute of Advanced Industrial Science and Technology (AIST; President: Ryoji Chubachi), in collaboration with Yoshihisa Harada (Associate Professor) of the Institute for Solid State Physics (Director: Masashi Takigawa), the University of Tokyo (President: Junichi Hamada) and others, used soft X-ray emission spectroscopy to reveal the detailed electronic structure of a cathode (positive electrode) material when charging and discharging a lithium-ion battery.
In this research, a unique cell with an organic electrolyte solution and a lithium metal anode (negative electrode) for analyzing the cathode of a lithium-ion battery was developed in order to measure soft X-ray emission spectra during charging and discharging. This cell was used to analyze the transfer of electrons to and from manganese ions in a lithium manganate positive electrode. Note that soft X-ray emission spectra were measured at the University of Tokyo Outstation BL07LSU of SPring-8. Clarifying the detailed charge-discharge mechanism of a lithium-ion battery composed of existing materials is expected to contribute to the development of next-generation electrode materials with even higher performance.
The results of this research have been published in the online version of Electrochemistry Communications, the letters journal of the International Society of Electrochemistry on November 25, 2014.
 |
|
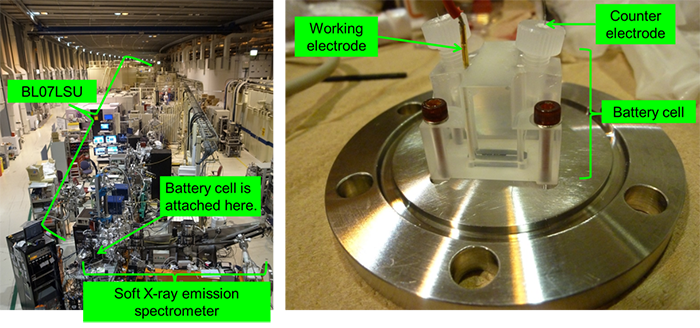
Appearance of the University of Tokyo Outstation BL07LSU in SPring-8 (left) and the developed cell for analysis (right) |
Lithium manganate (LiMn2O4), lithium cobaltate (LiCoO2), etc. have been widely used as cathode materials for lithium-ion batteries. However, the performance of these materials, such as charge-discharge capacity, is inadequate for large applications, including electric vehicles and stationary energy storage systems. Also, lower cost and higher cycle performance are desired.
In order to effectively improve the performance of these cathode materials, it is important to understand the charge-discharge mechanism of typical materials. Therefore, much research has been conducted to monitor the transfer of electrons to and from (reduction-oxidation (redox) reactions of) transition metal elements, such as cobalt (Co) and manganese (Mn), during charge-discharge reactions. Conventional X-ray absorption spectroscopy, which uses synchrotron radiation hard X-rays, provides element-selective information on which transition metal elements are reacting but cannot derive detailed information on electronic structure.
Meanwhile, applications of synchrotron radiation soft X-ray spectroscopy (soft X-ray absorption spectroscopy, soft X-ray emission spectroscopy, etc.), which provides more detailed information, have also been pursued. However, because they require the sample to be set in a vacuum, soft X-ray spectroscopic measurements could not be performed for cathodes or anodes during charge-discharge operations within an electrolyte solution. If such measurements were carried out, detailed knowledge of electronic structure could be obtained. If the electronic structure could be controlled by means such as element substitution so that redox reactions of oxygen in cathodes (generally considered redox-inactive) could also be used, then charge-discharge capacity would dramatically increase. Similarly, more stable or safer and longer-lived batteries should also be expected by some improvements based on the knowledge of electronic structure. Therefore, soft X-ray spectroscopic measurements of electrodes in lithium-ion batteries are desired.
AIST has been working to develop cathodes to improve the performance of lithium-ion batteries. Charge-discharge mechanisms of existing materials are critical to lead to ideas for developing novel electrodes of lithium-ion batteries. In order to clarify those mechanisms, AIST has focused on various analyses, such as computer simulations to reveal the mechanism of ion dynamics between and within electrodes, crystal structure analyses, and electronic structure analyses using hard X-ray absorption spectroscopy.
Recently, research had been conducted on the electronic structure of electrodes by using soft X-ray spectroscopy, which can provide a more-detailed analysis of the transfer of electrons from and to transition metal elements accompanying charge-discharge. For soft X-ray spectroscopy measurement, however, the electrode must be taken from the disassembled cell, preventing proper evaluation of the electronic structure of the electrodes during charge-discharge. Therefore, AIST worked to develop a soft X-ray spectroscopy technique that could be used during the charging and discharging of electrode materials.
This research was conducted with support from the Grant-in-Aid for Scientific Research (Japan Society for the Promotion of Science: Young Scientists (B) ) and the "Japan-U.S. Cooperation Project for Research and Standardization of Clean Energy Technologies (FY2010 to FY2014)" commissioned by the Ministry of Economy, Trade, and Industry.
In soft X-ray spectroscopy, soft X-rays must pass through a vacuum. Therefore, in order to measure samples under atmospheric pressure, the vacuum chamber and atmospheric pressure chamber must be separated with a thin-film window primarily composed of silicon nitride, which allows the passage of soft X-rays. Although this type of measurement technology was developed in recent years, there have been no examples in which a lithium-ion battery with organic electrolyte solution or its electrode materials have been measured.
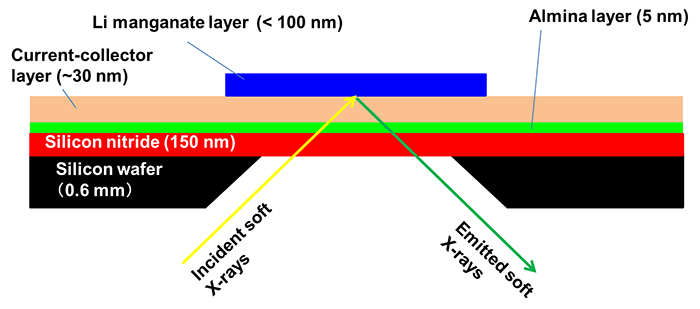
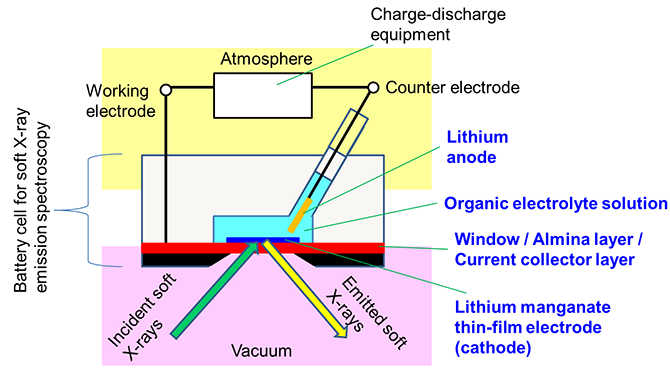
In this research, a multi-layered film consisting of the following sequence of layers was created: a silicon substrate coated with a silicon nitride window material (150-nm thickness), an alumina layer to improve adhesion to metal, and a metal current collector layer composed of two layers of titanium and gold. A thin film of lithium manganate was directly fabricated on top of that, with a thickness of the thin film of 100 nm or less. In addition, the central part of the silicon substrate was removed by chemical treatment to expose the silicon nitride window. Thus a unique thin-film electrode was prepared (Fig. 1). Using this thin-film electrode as the cathode in combination with a lithium negative electrode and a general organic electrolyte solution used in assessing lithium-ion batteries, a cell was developed that could perform soft X-ray emission spectroscopic measurements during charge-discharge operations (Fig. 2).
 |
|
Figure 1: Schematic illustration of the thin-film electrode |
 |
|
Figure 2: Schematic illustration of the cell |
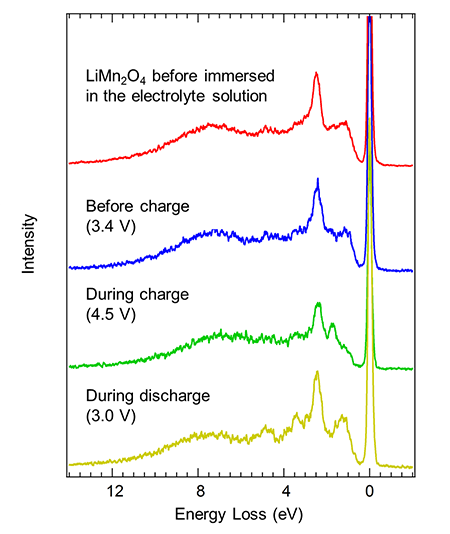
After charging and discharging the developed cell once, the soft X-ray emission spectra for the manganese were measured before the second charge (3.4 V), during the second charge (4.5 V), and during the second discharge (3.0 V). The values in the parentheses are the electric potentials during the measurements. Figure 3 shows the experimental results. The horizontal axis shows the energy difference between the incident soft X-rays and the X-rays emitted from the sample, in units of electron volts. Initially, two types of manganese, Mn3+ and Mn4+, coexisted in the lithium manganate thin film before it was immersed in the electrolyte solution. The emission spectrum before charging was in the same profile as the initial state, and the electronic structure of manganese in the first charge-discharge cycle changed reversibly and had returned to its original state. The spectrum during charging was very different than that before charging, and all of Mn3+ ions that coexisted with Mn4+ before charging should be oxidized to Mn4+ ions. In particular, the intensity of the peak near 8 eV originating from the bonding between manganese and oxygen was relatively larger than the peaks (1 eV to 6 eV) originating from the manganese 3d orbitals themselves, indicating that the bonding between oxygen and manganese became very strong during charging (in other words, for the Mn4+ state). Because the bonding strength between manganese and oxygen changed greatly as manganese was oxidized/reduced between the Mn3+ and Mn4+ states, the chemical bonding between manganese and oxygen is thought to fade as the charge-discharge cycle is repeated; this fade would be related to the deterioration in electrode performance.
Previously, crystal-structure analysis had clarified the expansion and contraction of the bonding distance between manganese and oxygen atoms caused by insertion and extraction of lithium. However, in the present research, soft X-ray emission spectroscopy was capable of evaluating the change in the strength of chemical bonding between the atoms from a viewpoint of electronic structure.
 |
|
Figure 3: Results of soft X-ray emission spectroscopy for manganese |
The spectrum during discharging closely resembled that before charging, and a reversible manganese redox reaction was found to take place even during the second charge-discharge cycle. However, the differences in the spectra during discharging and before charging suggested that the percentage of Mn3+ was higher during discharging, which corresponds to the greater tendency for manganese to be reduced during discharging, when the electric potential is low (3.0 V), than before charging (3.4 V). This slight change is hardly detected by measurements such as hard X-ray absorption spectroscopy, and soft X-ray emission spectroscopy was shown to be superior in this respect. Assuming that all of manganese ions during charging (4.5 V) has become Mn4+, the mean valence numbers of manganese are estimated to be Mn3.6+ before charging and Mn3.3+ during discharging by an analysis using the spectra before charging, during charging, and during discharging.
Thus, the present method revealed the redox reaction of manganese in a lithium manganate positive electrode, making it possible to obtain information related to manganese-oxygen bonding and the ratio of Mn3+/Mn4+, which had previously been difficult.
In order to obtain development guidelines for improving electrode performance, such as by elemental substitution, future research will also apply the present method toward other cathode materials to systematically reveal the correlation between charge-discharge cycle performance and chemical bonding between atoms. Also, the researchers will consider formulating development guidelines aimed at increasing the capacity and voltage of electrode materials and lowering their cost by using electronic-structure information obtained from the present method.