- Expected contribution to the rational design of insulation films, transparent electrodes, etc. -
Kengo Nishio (Senior Researcher), Hisao Nakamura (Senior Researcher) and Takehide Miyazaki (Leader) of the Nonequilibrium Materials Simulation Group, the Nanosystem Research Institute (Director: Tomohiko Yamaguchi) of the National Institute of Advanced Industrial Science and Technology (AIST; President: Ryoji Chubachi), have found that there is a structural universality in amorphous metal oxides independent of the kinds of metals and the ratios of the metal and oxygen, based on geometric consideration and first-principles calculation.
Amorphous metal oxides are important materials used for insulation films in transistors, memory chips, etc., and for the transparent electrodes of solar cells. Understanding of the material structure at the atomic level is required to suppress the variation of the material structure at the atomic level so as to reduce the size of devices to the nanometer level. In the present research, the arrangements of metal atoms and oxygen atoms are analyzed separately and it is predicted that the structure of an amorphous metal oxide is the superposition of a random packing structure of the metal atoms and that of the oxygen atoms.
This result is expected to contribute to the technical upgrading in designing materials for electronic devices. The details of this result are described in Physical Review Letters volume 111, 155502 (2013) of the American Physical Society.
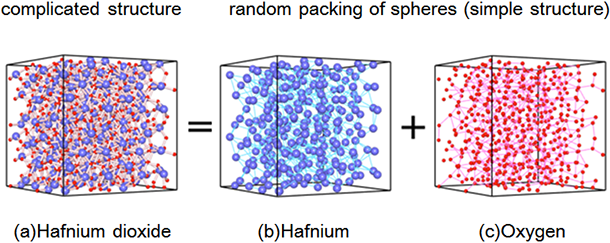 |
(a) Atomic arrangement of amorphous hafnium dioxide (also called hafnia) obtained by first-principles calculation
Blue and red spheres represent hafnium and oxygen atoms, respectively. The structure (a) is made up of a superposition of random packing structures of (b) hafnium and (c) oxygen, respectively. |
Metal oxides are important materials used in the insulation films of transistors and memory chips, and as transparent electrodes in solar cells. The amorphous structure is often used to obtain uniform films and to make the film forming process easier. In order to miniaturize devices to the nanometer scale aiming to reduce power consumption and to improve the performance of devices, it is necessary to create homogenous materials at the atomic level. Sufficient knowledge of the atomic structure of amorphous metal oxides would enable the more rational design of the process conditions that satisfy both the homogeneity and electric insulation of the materials. Unlike crystalline metal oxides, the atomic arrangement of amorphous metal oxides is very complicated, with no regularity, and thus a comprehensive understanding of the atomic structure of amorphous metal oxides has not been achieved.
AIST has been working on the development of simulation technology to accelerate the development of advanced materials and devices. In the case of amorphous materials, the structural information at the atomic level could not be precisely obtained solely through an experimental approach. Therefore, highly precise modeling of amorphous structures using simulation is required. Hitherto, the structural modeling of an amorphous material, aggregated metal encapsulated silicon clusters, was carried out and it was discovered that the structure similar to those of amorphous and liquid silicon could exist while the cluster structure was maintained. In addition, the stability of amorphous argon confined in pores with diameter of a few nanometers was studied and it was proposed that the most stable structure for the argon solid in the nano pores was not a crystalline structure but an amorphous structure. In the present study, the structures of amorphous metal oxides are systematically analyzed using geometric consideration and first-principles calculation so as to comprehensively understand the structure of amorphous metal oxides.
Elucidation of the amorphous structure is essentially one of the most difficult tasks in condensed matter physics. Figure 1 shows the conceptual difference between a crystalline structure and an amorphous structure. In crystals, atoms are periodically arranged (Fig. 1 (a)) and thus, their structures can be comparatively easily identified. However, amorphous structures (Fig. 1 (b)) have a disordered structure without periodicity in atomic arrangements unlike crystals. It is difficult to get detailed structural information except for regularity relating to the arrangement of nearest-neighbouring atoms, namely the number of surrounding atoms for a particular atom tends to be a definite value (short range order). In amorphous metal oxides, the number of the nearest oxygen atoms around a metal atom is different according to the metal’s type and its oxygen content. For example, alumina (Al2O3) has 4 and 5, titania (TiO2) and indium oxide (In2O3) both have 6, and zirconia (ZrO2) and hafnia (HfO2) both have 6 and 7. Thus, no common properties could be found for amorphous metal oxides in regard to short range order. Because of this, no comprehensive investigation of the respective material has been carried out until now.
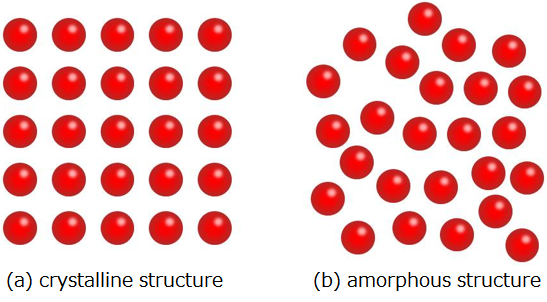 |
Figure 1 : Conceptual diagrams showing the difference between a crystal structure and an amorphous structure
The red spheres represent atoms. |
AIST investigated the arrangement of the second nearest-neighboring atoms (medium range order) to clarify whether there is a common atomic arrangement in amorphous metal oxides regardless of the difference in the metal’s type and oxygen content and what that possible arrangement is. For this purpose, AIST reverted to the geometric consideration of the crystalline structure and constructed a methodology to consider the principle governing the structure of amorphous metal oxides. First, numerous substances composed of one kind of atom have crystalline structures that resemble the crystalline packing of spheres, such as a face-centered cubic lattice, a body-centered cubic lattice, etc. Next, both the arrangement of metal atoms and that of oxygen atoms in crystalline metal oxides resemble crystalline packing structures or structures close to them. For example, a copper oxide (Cu2O) crystal can be regarded as a superposition of a face-centered cubic lattice of copper atoms and a body-centered cubic lattice of oxygen atoms. Generalizing this result, it is expected that both the metal atoms and oxygen atoms respectively form structures similar to the random packing of spheres in amorphous metal oxides. In other words, the structure of amorphous oxides is a superposition of random packing of metal atoms and that of oxygen atoms.
For confirmation of above mentioned idea, seven metals, titanium (Ti), zirconium (Zr), hafnium (Hf), copper (Cu), aluminum (Al), gallium (Ga) and indium (In), were selected and the first-principles calculations of the amorphous structures for their oxides (TiO2, ZrO2, HfO2, Cu2O, Al2O3, Ga2O3 and In2O3) were carried out.
Both in crystalline and amorphous states of metal oxides, nearest neighbors of metal atoms are oxygen atoms. Therefore, to investigate the medium range order by analyzing the second nearest neighbors, the researchers decompose the structure of metal oxides into the arrangement of metal atoms and that of oxygen atoms. Then they investigated how metal (oxygen) atoms are arranged around a metal (oxygen) atom. In Fig. 2, the icosahedron arrangement of metal atoms and that of oxygen atoms in Al2O3 are illustrated. It is known that random packing of spheres contains pentagonal bipyramidal structures as shown in Fig. 3. As icosahedral structures consists of pentagonal-bipyramid structures, it is strongly suggested that the structure of amorphous metal oxides are the superposition of metal and oxygen respectively forming structures similar to the random packing of spheres.
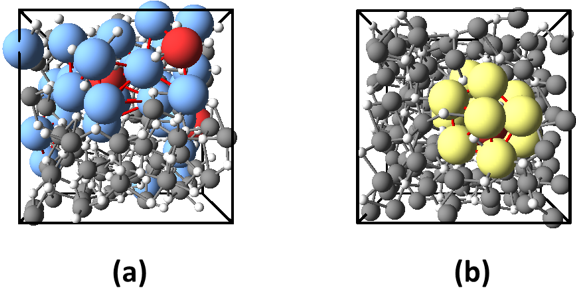 |
Figure 2 : Icosahedral arrangements in amorphous Al2O3 obtained by the first-principles calculation
Large colored spheres show the icosahedral arrangement of (a) aluminum atoms and (b) oxygen atoms. |
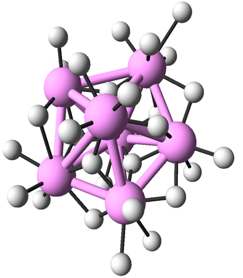 |
|
Figure 3 : Pentagonal-bipyramid structure (Large colored spheres) |
A polyhedron surrounded with bisector planes between nearest-neighboring metal atoms and those between nearest-neighboring oxygen atoms (this is called the Voronoi polyhedron) is constructed on a computer to quantitatively investigate pentagonal-bipyramid structures in the amorphous metal oxides and the numbers of sides of surfaces forming the Voronoi polyhedron were counted to investigate the distribution of the surface shapes (Fig. 4). If there are a number of pentagonal-bipyramid structures, the Voronoi polyhedra are formed with a number of pentagons. As an example of a random packing structure of spheres, the amorphous structure of a particle system, in which particles interact according to the Lennard-Jones potential, was similarly analyzed (Fig. 4 (i)). As clearly shown in Fig. 4(a) to (g), pentagonal shapes were the most frequent in the shape of surfaces constructing the Voronoi polyhedron for both metals and oxygen, regardless of the metal type or oxygen content, and the appearance frequencies declined in order from hexagonal → tetragonal → heptagonal → trigonal → octagonal. This is the same result as that shown with the Lennard-Jones potential system in Fig. 4(i). It was concluded that the structure of amorphous metal oxides is formed as a combination of random packing structures of both metal and oxygen atoms.
In other words, the present research has identified that universal structure of the substances. Figure 4(h) shows the results of an oxide of semiconductor Si (SiO2). Tetragonal shapes are the most frequent in the Voronoi polyhedron of the silicon network, and only the oxygen atoms form a random packing structure. A strong covalent bond between silicon and oxygen is considered to be the main cause.
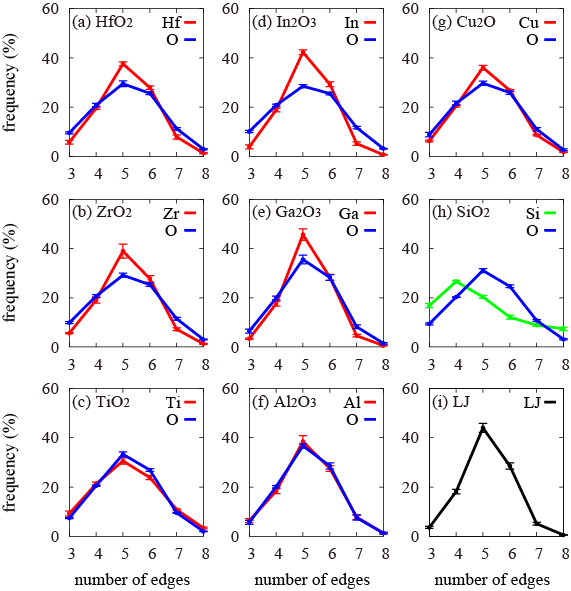 |
Figure 4 : (a) to (g) Respective edge number distribution in Voronoi polyhedrons of metals and oxygen in the amorphous metal oxides
For comparison, analysis results of (h) the amorphous structure in an oxide of semiconductor Si (SiO2), and of (i) the amorphous structure, in which particles interact according to a Lennard-Jones potential, are shown. All (a) to (g) are metal oxides, but (h) is a result for an oxide of semiconductor with different characters and the curve is shown in green to differentiate it from (a) to (g). |
The reason that the structure of amorphous metal oxides is formed as a superposition of the random packing structure of metal atoms and that of oxygen atoms can be explained qualitatively as follows. Charge transfer occurs in metal oxide from metal to oxygen and, as a result, metal atoms are charged positively and oxygen atoms are charged negatively, thus the metal and oxygen atoms bind with ionic bonds. Looked at from the network of metal atoms, the role of oxygen atoms may be considered as uniform negative charges. The negative charges prevent the breaking of the metal atom network by reducing the repulsion of positive charges on the metal atoms. Conversely, the metal atoms are considered to be uniform positive charges for the network of oxygen atoms. The positive charges reduce the repulsion between the negative charges of the oxygen atoms and prevents the breakage of the oxygen atom network. The factor dominating the medium range order is considered to be the repulsive force with spherical symmetry between the positive charges of the metal atoms and the repulsive force between the negative charges of the oxygen atoms, and the forces are considered to cause the random packing of the atoms.
Utilization of the present findings regarding the medium range order is expected to give more accurate modeling of amorphous metal oxide structures and to give better suppression of accuracy dispersion than was previously the case. In future, further development to enable rational modeling of the atomic structure of various amorphous metal oxide materials such as insulation films used in nanometer-sized electronic devices and transparent electrodes of solar cells will be carried out.