Mayako Kutsukake (Researcher), Takema Fukatsu (Leader) and others, Symbiotic Evolution and Biological Functions Research Group, the Bioproduction Research Institute (Director: Yoichi Kamagata) of the National Institute of Advanced Industrial Science and Technology (AIST; President: Tamotsu Nomakuchi), have discovered a novel phenomenon that in galls (plant-made nests) formed by some aphid species, the inner gall wall promptly absorbs and removes watery waste that can kill the aphids when accumulated within the galls.
Aphids take plant phloem sap as the sole food source and excrete a large amount of watery waste (honeydew). The symbiotic relationship between ants and aphids is well known such that ants take the honeydew excreted by aphids and the aphids are protected from outside enemies by ants. A part of aphid species form galls on host plants, and sustain a social life therein. In most cases, soldier nymphs discard honeydew through the openings of galls. However, some social aphids form completely closed galls having no opening, but how the honeydew is handled within completely closed galls has remained unknown. This research revealed that the aphids sophisticatedly manipulate the plant morphology and physiology, and induce their galls to have a honeydew-absorbing property. This study will shed light on understanding of morphological formation and functional modification of plants.
The results were published online on November 14, 2012 (JST) in Nature Communications, a British scientific journal.
 |
|
Figure 1 : Galls of various aphids
(a) Gall of Nipponaphis monzeni. (b) Gall of Tuberaphis styraci. (c) Gall of Ceratovacuna nekoashi. (d) Galls of Ceratovacuna japonica. Galls (a) and (c) are completely closed and (b) and (d) are open galls. |
Insects have acquired ingenious biological functions in order to adapt to various environments in the process of evolution. Humankind has succeeded in industrial applications of such insects, like silkworm and honeybee. However, because a large variety of insects—far larger than 1,000,000 species—exist on the earth, numerous interesting biological phenomena, novel biological functions, and genetic resources, therefore, remain undiscovered.
Meanwhile, most organisms do not exist independently, but rather have evolved through the complicated and sophisticated interactions among organisms. Such interactions among organisms range from close ones to loose ones. In the close interactions, for example, organisms such as insects sometimes have effects on the physiological conditions of other organisms such as plants, and manipulate the morphology and physiology for their own sake. Such a phenomenon is interesting not only from basic biological aspects, but also from practical aspects for development of technologies controlling morphology, development, and biological functions of plants by external factors. Therefore, investigation to elucidate the phenomenon is awaited.
AIST is conducting studies on various insects focusing on interesting biological phenomena accompanying close interactions between organisms. Concerning gall-forming social aphids, AIST has striven to elucidate novel, complicated, and sophisticated biological functions of the aphids (AIST press releases on July 27, 2004 and February 25, 2009). This discovery was achieved as part of these studies. The field experiments in this study were formally approved by AIST and conducted complying with the rules of AIST.
Nipponaphis monzeni (N. monzeni) forms galls having a cavity on Distylium racemosum evergreen trees, wherein more than 2,000 aphids sometimes live in groups. Galls functions as not only protective walls from enemies and environmental changes, but are also food supply sources such that aphids can suck plant phloem sap only by piercing the inner walls with their stylets.
Gall shapes are quite different depending on the aphid species (Fig. 1), suggesting aphids are involved in the special morphogenesis of plants. N. monzeni forms completely closed galls without openings (Fig. 1a). Aphids of N. monzeni are isolated from the outside environment during at least two years until the galls mature and form an opening and winged aphids migrate to the secondary host plants. A problem is how they cope with their excreted honeydew in the completely closed galls. In general, aphids excrete a large amount of sugar-rich honeydew. The enigmas here are why these social aphids are not drowned and killed by accumulated honeydew in closed galls and what mechanisms the aphids have in order to avoid such a risk.
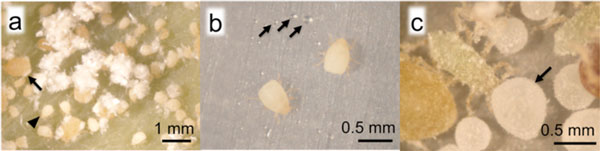
Galls of N. monzeni were examined. Solid wastes such as corpses, shed skins, and secreted powdery wax were found, but no accumulation of excreted honeydew was observed in the galls, although hundreds of aphids live therein (Fig. 2a). However, when aphids in the galls were placed on an artificial feeding system, a number of honeydew droplets were observed around the aphids on the next day (Fig. 2b). It was thus revealed that honeydew was not accumulated within galls although the aphids excreted honeydew.
 |
|
Figure 2 : Aphids living in galls
(a) N. monzeni in completely closed galls. Arrows indicate mature aphids and arrowheads indicate the first-instar soldier nymphs. Flocculate substances are solid waste products of aphids, secreted wax. (b) N. monzeni placed on the artificial feeding system. Arrows indicate honeydew. (c) Tuberaphis styraci that forms open galls. An insect at the center is a soldier nymph cleaning and removing honeydew (indicated with arrows). |
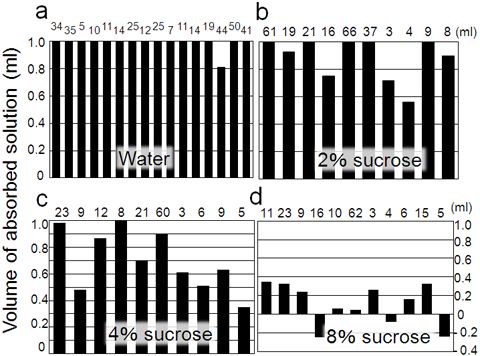
A field experiment was conducted to confirm if honeydew excreted by aphids is absorbed by gall tissue. A small hole was bored on the gall wall of N. monzeni, 1 ml of distilled water or sucrose water was injected through the hole, and then the hole was closed with a woodwork adhesive. After 20 hours, the galls were collected and the amount of the solution remaining in them was examined. As a result, distilled water was completely absorbed and disappeared from almost all galls (Fig. 3a). Sucrose water was also absorbed, but absorption efficiency decreased as the sucrose concentrations increased (Figs. 3b - 3d). Analysis of sugar concentration in honeydew of N. monzeni showed that it was lower than 0.5%, which is the level to be sufficiently absorbed by the gall tissue.
 |
|
Figure 3 : Results of water absorption experiments using galls of N. monzeni
(a) Distilled water in galls of N. monzeni, 16 galls. (b) 2% sucrose water in galls of N. monzeni, 10 galls. (c) 4% sucrose water in galls of N. monzeni, 10 galls. (d) 8% sucrose water in galls of N. monzeni, 11 galls.
Each solution (1 ml) was injected to one gall. The number above the bar graph indicates the volume of each gall. |
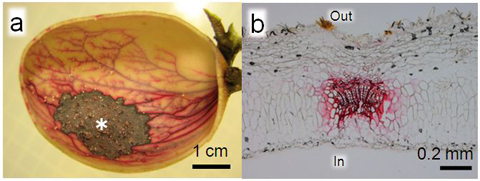
The route of the aqueous solution absorbed by the inner gall wall was visualized by safranin staining. The results showed that the absorbed safranin solution was removed via vascular bundle of the plant. (Fig. 4)
 |
|
Figure 4 : Tracking experiment for absorbed aqueous solution
(a) N. monzeni gall inside which absorbed the safranin solution (safranin solution was applied to the point indicated by *). Gall tissue in the periphery of the point (*), where the safranin solution had been absorbed, was stained dark red. The absorbed safranin solution was transported and dispersed within the gall tissue, and stained the veined structure in red. (b) Gall tissue section. Vascular bundles were stained in red. In, inner side of the gall; Out, outer side of the gall. |
On the other hand, galls of many social aphid species have one or more openings and soldier nymphs push and discard honeydew droplets with their heads through the openings, thereby keeping the gall inside clean (Fig. 2c). Tuberaphis styraci (T. styraci) forms open galls on Styrax obassia deciduous trees (Fig. 1b). The researchers conducted similar water absorption experiments with galls of T. styraci and found that they never absorbed water.
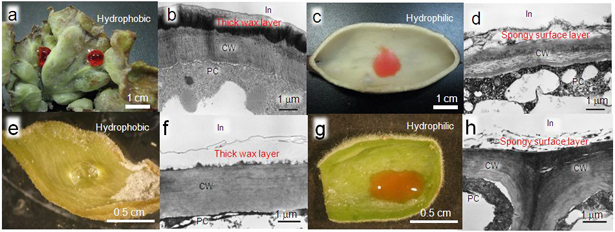
The inner gall walls of N. monzeni and T. styraci were further examined. The inner surface of open galls of T. styraci was coated with a thick wax layer and it was hydrophobic (Figs. 5a and 5b). Meanwhile, the inner surface of completely closed galls of N. monzeni had a spongy layer and it was hydrophilic (Fig. 5c and 5d). In the case of galls of other aphid species, whereas the inner surface of open galls of Hamamelistes miyabei were hydrophobic and coated with a thick wax layer, the inner surface of completely closed galls of Nipponaphis distyliicola were hydrophilic and spongy. From these results, it was considered that the structure of inner gall wall probably relate to the water-absorbing property of aphid galls.
 |
|
Figure 5 : Characteristics of inner gall walls of various aphids
(a) and (b) show the galls of T. styraci (open galls).
(c) and (d) show the galls of N. monzeni (completely closed galls).
(e) and (f) show the galls of C. japonica (open galls).
(g) and (h) show the galls of C. nekoashi (completely closed galls).
(a), (c), (e), and (g) show the results when a drop of solution is added on the inner gall wall. (Differences in liquid color have no meaning.)
(b), (d), (f), and (h) show the cross sections of the inner gall wall observed by a transmission electron microscope.
CW, cell wall; In, inner side of the gall; PC, plant cell |
Moreover, galls formed by different aphid species on the same plant were compared. Both Ceratovacuna nekoashi (C. nekoashi) and Ceratovacuna japonica (C. japonica) form galls on Styrax japonicus deciduous trees (Fig. 1c and 1d). These species are classified into the same genus Ceratovacuna, and have similar ecological characteristics. An important difference between the two is that C. japonica forms open galls, while C. nekoashi forms completely closed galls. Furthermore, soldier nymphs of C. japonica perform gall cleaning, but those of C. nekoashi do not perform gall cleaning.
The galls of the two species were more closely examined. In the open galls of C. japonica, honeydew was accumulated at a high level in the galls, and the inner wall was hydrophobic and coated with a thick wax layer (Fig. 5e and 5f). On the other hand, in the completely closed galls of C. nekoashi, no honeydew accumulation was observed in the galls, and the inner wall was hydrophilic and spongy (Fig. 5g and 5h). Furthermore, when distilled water was injected into the completely closed galls of C. nekoashi, water was completely absorbed. From these results, it was indicated that the water absorbing property is not determined by plant species, but by gall-forming aphid species. In addition, clear patterns were consistently observed in this study: completely closed galls absorb water, but open galls do not absorb water.
From an ecological point of view, open galls have a disadvantage that they are vulnerable to enemy invasion, but have an advantage that colony hygiene can be maintained by discarding honeydew out of the galls. On the other hand, completely closed galls have an advantage that enemy invasion can be easily prevented, but have a disadvantage that honeydew cannot be disposed out of the galls. Therefore, aphids inhabiting completely closed galls have solved the hygienic problem by manipulating the plants and inducing the water-absorbing property. As far as the researchers examined in this study, all completely closed galls have the water-absorbing property, indicating that the two traits are closely linked and essential for realization of long-term su-gomori life (reclusive life) in the closed nest.
These results are novel findings concerning the insects' manipulation of plant morphogenesis and physiology for their own sake. It is also interesting in view of the control of plant traits by external factors.
The researchers will further elucidate this phenomenon focusing on metabolic conversion of sugars and amino acids taken from the absorbed honeydew, and on the possibility of recycling of these substances in plants. Moreover, they will analyze gene expression in aphid galls by using a next-generation sequencer to understand the molecular mechanisms underlying gall formation.