Update(MM/DD/YYYY):06/15/2012
Discovery of Symbiotic Bacteria Mediating Insecticide Resistance to Pest Insects
- Overturns conventional understanding that insecticide resistance is determined only by the pest Insect’s own genes -
Points
-
Discovery of symbiotic bacteria influencing insecticide resistance, a critical characteristic for pest insect control
-
Bean bugs, a kind of pest insect, develop insecticide resistance by acquiring insecticide-degrading bacteria found in soil.
-
Presenting a new perspective for planning how to control pest insects
Summary
Yoshitomo Kikuchi (Researcher), the Environmental Biofunction Research Group, Takema Fukatsu (Leader), the Symbiotic Evolution and Biological Functions Research Group, and others of the Bioproduction Research Institute (Director, Research Institute: Youichi Kamagata) of the National Institute of Advanced Industrial Science and Technology (AIST; President: Tamotsu Nomakuchi), in collaboration with Masahito Hayatsu (Senior Researcher) and Kanako Tago (Researcher) of Environmental Biofunction Division, National Institute for Agro-Environmental Sciences (NIAES; President: Kiyotaka Miyashita), Atsushi Nagayama (Researcher) of Plant Disease and Insect Pest Management Section, the Okinawa Prefectural Agricultural Research Center (OPARC; Director General: Moriaki Sakamoto), and others, have discovered that bean bugs (Riptortus pedestris), a kind of pest insect that attacks soybean crops and is difficult to control, may develop insecticide resistance by acquiring insecticide-degrading bacteria from soil and allowing them to live symbiotically in their bodies.
To date, insecticide resistance has been reported in approximately 500 types of pest insects around the world, causing serious problems for agriculture and public health. It has conventionally been believed that insecticide resistance is determined only by the genes of pest insects themselves. However, this new discovery overturns this conventional understanding, presenting a new perspective on the evolution of insecticide resistance in pest insects and for planning strategy to control pest insects.
The results will be published online in a US academic journal, Proceedings of the National Academy of Sciences USA, on April 24, 2012 (JST).
 |
|
Figure 1 : A bean bug on a soybean leaf |
Social Background of Research
Though there are problems of the environmental load and residual agricultural chemicals, insecticides have become increasingly critical for stable food supply in recent years, as climate change and population growth have caused serious concern about global food shortage. The use of insecticides, moreover, has become indispensable to control hematophagous sanitary pest insects such as anopheline mosquitos (a malaria vector) and tsetse flies (a sleeping disease vector) as well as household insects like termites and cockroaches.
It has long been known, however, that if a single insecticide is used continuously, pest insects frequently emerge that have insecticide resistance. So far it has been reported that over 500 types of agricultural, sanitary, household, and other pest insects have developed resistance to insecticides, which is a significant problem. Various examples have been reported as mechanisms of resistance, including enhanced detoxification capability and structural changes in target proteins, but the conventional understanding is that in each case insecticide resistance is a characteristic regulated by the genes of the insect itself. Because many pest insects possess symbiotic microorganisms inside their bodies, the possibility that these symbiotic microorganisms may affect the insecticide resistance of their host insects has been suggested but never proven up to now.
The emergence of insecticide-resistant pest insects, similar to the problem of multidrug-resistant pathogens in medical institutions, is an endless battle between human beings developing insecticides and pest insects developing resistance. Because the development of new insecticides takes a great deal of time and money, it is very important to prevent insects from developing resistance, and the biggest issue to address for that purpose is to understand the mechanism of resistance development.
History of Research
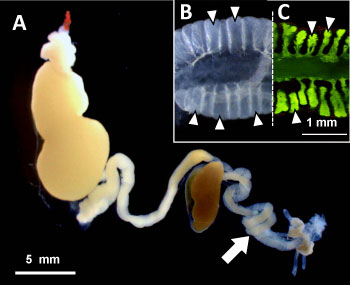
AIST has been conducting a variety of research looking at sophisticated bio-functions of symbiotic microorganisms living inside insect bodies (AIST press releases on March 25, 2004 and November 19, 2010). In recent years, research has focused on stinkbugs, which includes many pest insects that are difficult to control, and in the process, bean bugs (Fig. 1), known as soybean pests, have been found to have a unique microbial symbiosis. The alimentary tract of a bean bug has many sac-like structures called crypt (Fig. 2B), where bacterial symbionts of the genus Burkholderia live (Fig. 2C). In most types of insects, symbiotic bacteria are transmitted directly from mother to offspring (vertical transmission), but in the case of bean bugs, hatchlings acquire the symbiotic Burkholderia from environmental soils every generation (environmental acquisition).
NIAES has been conducting research on various functions of soil microorganisms with the aim of using them to improve agricultural land or remediate polluted environments. One beneficial function of microorganisms is the ability to degrade and clean up chemicals, including insecticides. NIAES has isolated and identified various insecticide-degrading bacteria, including many strains of Burkholderia.
The recent results were based on cumulative research on symbiotic microorganisms at AIST and cumulative research on insecticide-degrading bacteria at NIAES, combining these lines of research and developing them further. In addition, the present research, especially by OPARC, studied insecticide-degrading bacteria infection status in field populations of stinkbugs.
This research was support by the “Program for Promotion of Basic and Applied Researches for Innovations in Bio-Oriented Industry” of the Bio-oriented Technology Research Advancement Institution of the National Agriculture and Food Research Organization.
 |
|
Figure 2 : Digestive tract and symbiotic organ of the bean bug |
(A) Digestive tract: Arrow indicates symbiotic organ.
(B) Enlarged image of symbiotic organ: A number of crypts (indicated by triangles) are developed,
which are full of the symbiotic bacteria Burkholderia.
(C) Crypts filled with Burkholderia visualized by green fluorescent dye. |
Details of Research
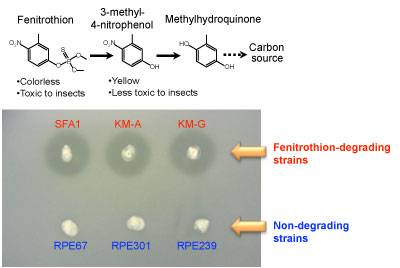
The insecticide fenitrothion, an organophosphate compound, is widely used around the world. It has been reported that various soil bacteria can degrade fenitrothion and use it as a sole carbon source. Through the action of degrading bacteria, fenitrothion is decomposed into 3-methyl-4-nitrophenol, which has little toxicity to insects, and then goes through multiple steps before being used as a carbon source (Fig. 3, top). Fenitrothion-degrading bacteria occur infrequently in cropland soils, but successive applications of fenitrothion increase their concentration.
 |
|
Figure 3 : Degradation of fenitrothion by Burkholderia |
(Top) Fenitrothion degradation pathway
(Bottom) Degrading activity in a culture medium containing fenitrothion
Halos are formed around colonies of degrading bacteria by the degradation of fenitrothion. |
Burkholderia, which are symbiotic bacteria of the bean bug, ordinarily live in environmental soil. As bean bug nymphs grow, they acquire Burkholderia orally and each generation newly establishes the symbiotic relationship. Because stinkbugs with symbiotic Burkholderia have much larger body sizes and numbers of eggs laid than those without, it is believed that Burkholderia play an important role in the nutritional metabolism of the host stinkbugs.
When Burkholderia strains were isolated from cropland soils from several regions and from the stinkbug species living there and were investigated, the Burkholderia isolated from the soil included strains that degrade fenitrothion, though in small numbers (Fig. 3, bottom).
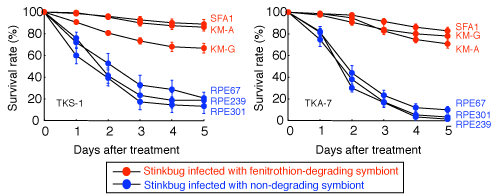
Bean bug specimens were infected with these fenitrothion-degrading Burkholderia strains (fenitrothion-degrading bacteria) or with Burkholderia strains incapable of degrading fenitrothion (non-degrading bacteria) and the effects on the host were compared. The results showed no significant difference in symbiotic bacteria infection rates, host survival rates, growth rates, body sizes, etc. between bean bugs infected with fenitrothion-degrading bacteria and those infected with non-degrading bacteria. However, the bean bugs infected with fenitrothion-degrading bacteria had much greater resistance to fenitrothion than did the bean bugs infected with non-degrading bacteria (Fig. 4). These results clearly demonstrate that the bean bugs had acquired insecticide resistance by being infected with insecticide-degrading bacteria.
 |
|
Figure 4 : Survival rates of bean bugs after fenitrothion treatment |
Almost none of the specimens died if they were infected with fenitrothion-degrading bacteria.
Results are shown for two bean bug lines: TKS-1 (left) and TKA-7 (right). |
Infection studies conducted on 846 bean bug specimens from various parts of Japan detected no fenitrothion-degrading bacteria (0%). A similar infection study was conducted on rice bugs (Leptocorisa chinensis), a kind of pest insect attacking rice plants, and oriental chinch bugs (Cavelerius saccharivorus), one that attacks sugarcane. (Both of these species have symbiotic relationships with Burkholderia.) This study found infection with fenitrothion-degrading bacteria only in some populations of oriental chinch bugs (approximately 8%). It was determined, in other words, that the fenitrothion-degrading bacteria infection rate is ordinarily low among field populations of stinkbugs in Japan. It is thought that, because applications of fenitrothion are limited to about one to three times a year on Japanese cropland, the concentration of fenitrothion-degrading bacteria in much arable soil is below the detection threshold.
When fenitrothion was applied experimentally (once a week for a total of four times) to soils taken from cropland, the concentration of fenitrothion-degrading bacteria in the soil increased, and more than 80% of the isolated and cultured bacteria showed fenitrothion degradating activity. Soybean plants were then planted in the soils where fenitrothion-degrading bacteria had been increased this way, and bean bug nymphs were raised there. More than 90% of the insects acquired fenitrothion-degrading bacteria by the time they reached adulthood. This indicates the possibility that successive applications of insecticide not only increase insecticide-degrading bacteria in soil but also promote infection with degrading bacteria in stinkbugs and thus help the pest insects to develop insecticide resistance.
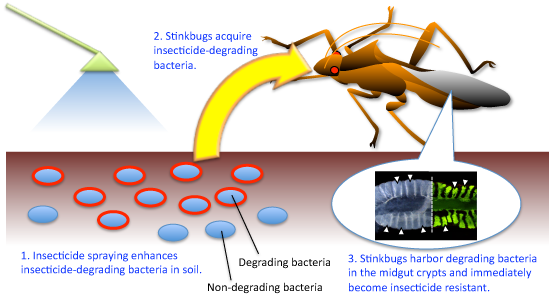
Based on these results, it is thought that the insecticide resistance with the help of symbiotic bacteria Burkholderia in pest insects is developed through processes described below (Fig. 5).
(1) Successive applications of insecticide enhance the propagation of insecticide-degrading bacteria in soil.
(2) Stinkbugs acquire these insecticide-degrading bacteria from the soil and live symbiotically with them.
(3) Stinkbugs harboring insecticide-degrading bacteria develop insecticide resistance.
 |
|
Figure 5 : Scheme of the development of symbiont-mediated insecticide resistance in pest insects |
The results demonstrate for the first time that symbiotic microorganisms can be involved in insecticide resistance in pest insects. It has been believed that the development of insecticide resistance is the result of genetic mutations in the pest insect itself, that resistant individuals appearing in a population of pest insects are selected by the use of insecticide, and that the number of such resistant insects in a population thereby gradually increased and emerged. The present discovery of the mechanism of the development of insecticide resistance with the help of symbiotic bacteria does not contradict the conventional insecticide resistance development model, but rather suggests a novel model to go along with it.
Future Plans
The researchers will work on decoding the entire genome of insecticide-degrading Burkholderia and will use a next-generation sequencer to perform a comprehensive analysis of expressed genetic changes in host stinkbugs before and after Burkholderia infection. Through these research efforts, they aim to elucidate what effect insecticide resistance, when it results from infection with symbiotic bacteria, has on the genetic expression and metabolic system of the host stinkbugs, and to clarify the molecular basis of this important phenomenon, both in basic and applied aspects.
Examples of important applied research that needs to be done include evaluating how much application of insecticide leads to the accumulation of insecticide-degrading Burkholderia in cropland soil and to the infection of stinkbugs with degrading bacteria. The researchers will study this question at experimental cropland and seek to clarify the effect that microbial community behavior in the soil has on the environmental adaptation of host pest insects.
Elucidating the mechanism by which symbiotic microorganisms boost insecticide resistance in pest insects could lead to the development of novel technologies to prevent insecticide resistance from even emerging in those insects. From that perspective, the researchers intend to proceed with their research.