Shinya Honda (Leader), Molecular and Cellular Breeding Research Group, the Institute of Biological Resources and Functions (Director: Masanao Oda) of the National Institute of Advanced Industrial Science and Technology (AIST; President: Tamotsu Nomakuchi) and his colleagues modified a protein that is used in the purification of therapeutic antibodies (so-called antibody drugs) based on molecular design and improved the stability and affinity of the protein.
Protein G, which has affinity for the constant regions of various antibodies, was modified based on molecular design. The modification improved the key properties related to purification of antibodies, such as heat resistance, chemical resistance, resistance to proteolysis, affinity for antibodies, and pH response. Protein G has already been used for purifying antibodies, but only at a laboratory scale. As the engineered Protein G has improved durability and enables the purification of antibodies under moderate conditions, the protein is feasible for their production at an industrial scale, too. The engineered Protein G is expected to be an effective purification tool in the manufacture of antibody drugs.
The details of this study will be presented on March 25 (local time) at the 2nd Annual International Congress of Antibodies, 2010 (ICA-2010) held in Beijing, China, on March 24-26, 2010.
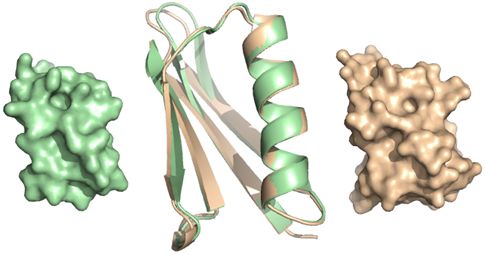 |
Figure Three-dimensional structures (determined by X-ray crystallography) of the engineered Protein G (right, orange) and wild-type (unmodified) Protein G (left, green)
The superimposed image (center) of the structures shows that the main chain structure of the engineered molecule is almost unchanged. |
Antibody drugs have higher efficacy and milder side effects than conventional low-molecular weight drugs and thus have a high rate of successful development. Their use is increasing at a high rate of about 50% a year on average. Today, the market in Japan has reached 130 billion yen. As antibody drugs are expensive, lowering their price is a matter of social need and there is a demand for technologies to lower the production cost.
Of the processes required to manufacture antibody drugs, the most costly is purification. At least half of the cost of purification lies in affinity chromatography (the process of separating and purifying a target substance by utilizing affinity between substances). Affinity chromatography is indispensable for increasing the purity of antibody drugs and ensuring their quality. Today, Protein A, a protein derived from a certain bacterium, is used as an affinity ligand (a molecule that has affinity for antibodies) to produce the majority of approved drugs, as it has affinity for the constant regions (i.e. the common parts in the molecular structure) of the antibodies. Protein A has superb properties, such as the ability to recognize and bind to antibodies specifically, but is a main source of high purification costs. Therefore, the development of an alternative affinity ligand has been awaited.
AIST has developed molecular design technologies for modifying proteins so as to gain the desired properties. We have published details of the creation of the “smallest protein,” chignolin, which consists of 10 amino acid residues that form a specific 3D structure and have undergone cooperative structural transition (AIST press release on August 10, 2004). We have also published a study on the crystalline structure and stabilizing modification of chignolin (the study appeared in the pages of “Latest Research” on the website of AIST, dated October 27, 2008).
Using originally developed molecular design programs, we aimed to modify Protein G, which has affinity for the constant regions of antibodies, and to develop an engineered protein with superb properties including heat resistance, chemical resistance, resistance to enzyme decomposition, affinity for antibodies, and pH response.
Some of the present results are fruits of the project entrusted by the New Energy and Industrial Technology Development Organization (NEDO) entitled “Development of new functional antibody technologies/Development of high performance separation technologies for antibodies (fiscal years 2006 to 2010)”.
Human beings have several kinds of antibodies. The present mainstream of antibody drugs is focused on immunoglobulin G (IgG) class. There are four subclasses of human IgG: IgG1, IgG2, IgG3, and IgG4. Protein A, which has been used to purify antibody drugs, has affinity for three of these but not for IgG3. In contrast, Protein G has affinity for all four subclasses.
Only in terms of this property, Protein G is a better affinity ligand than Protein A. However, Protein G lags behind Protein A in terms of strength of affinity and pH response. These disadvantages have impeded the use of Protein G in the industrial production of antibody drugs, despite the fact that it is widely used in laboratories. We designed an engineered Protein G molecule that has better antibody affinity and other affinity-ligand molecular properties than Protein A without losing the original affinity of the protein for all four subclasses.
The engineered protein was developed in two stages. First, the amino acid sequences were designed to stabilize the molecular structure of Protein G (first generation). Then the stabilized Protein G molecules were further modified to improve molecular recognition properties such as affinity (second generation).
In the first-generation design, the amino acid sequences were generated by inputting the atomic coordinates of wild-type (unmodified) Protein G structure into an originally developed molecular design program. According to the output from the program, several types of engineered Protein G were synthesized. All engineered Protein G molecules showed increases of 7 to 13 °C in heat resistance, 1.4 to 1.6 times increases in chemical resistance against denaturants, and 4 to 14 times increases in resistance against proteases. Because increases in the stability of a protein molecule lead to improved durability of the protein as an affinity ligand in the purification process, protein stabilization is a key to developing a low-cost system of antibody-drug production. The 3D structure of one of the engineered proteins was then determined by X-ray crystallography. In parts other than the engineered region, the atomic coordinates (3D structure of the molecule) of the protein were almost the same as those of wild-type Protein G (see Figure).
In the second-generation design, the computer model structure of a complex of the first-generation engineered Protein G and the antibody IgG1 was built from their 3D structures. From this model, we redesigned the amino acid sequence to improve the affinity by conducting computer simulations including that of electrostatic repulsion at the contact interface between the engineered Protein G and IgG1 within the complex. The redesigned engineered Protein G was synthesized and its properties were examined. The antibody affinity at neutrality was found to be 11 times higher, and the pH response (evaluated by comparing the affinities at neutral and acidic conditions) was 18 times that of wild-type Protein G.
Then the behaviors of the affinity chromatography, in which the second-generation engineered Protein G is used as an affinity ligand, were investigated by using an already approved antibody drug. The engineered protein was found to shift the pH of the peak elution of the adsorbed antibody drug to the neutral side, from pH 3.1 to pH 4.1. Because purification under moderate conditions reduces the risk of antibody-drug degradation during production, it is indispensable to enable purification to be performed under milder conditions in order to secure the quality of antibody drugs. Furthermore, the second-generation engineered Protein G was shown to have sufficient affinity for all subclasses of human IgG.
The following table summarizes the properties of Protein A, unmodified Protein G, and the second-generation engineered Protein G developed in this study. In all items, the engineered Protein G was superior to unmodified Protein G, showing that the molecular design was highly effective. The engineered Protein G was superior to Protein A in several properties and has affinity for all IgG subclasses; it is thus likely to be a feasible alternative to Protein A, which is the ligand most widely used in the purification process during the manufacture of antibody drugs.
Studies on the developed engineered Protein G will be continued with the aim of practical implementation of this molecule in the purification process during antibody-drug production. The technology will be transferred to private companies. The molecular design technology constructed will be deployed in the fields of drug design and drug production management.