- Enabling the contribution to the early detection and development of curative technologies for cancer -
Yoshifumi Jigami (Director and Team Leader), Takehiko Yoko-o (Senior Researcher), and Mariko Umemura (Technical Staff) of the Glycobiosynthesis Team of the Research Center for Glycoscience of the National Institute of Advanced Industrial Science and Technology (AIST; Hiroyuki Yoshikawa, President), and Morihisa Fujita (Researcher) of the Japan Society for the Promotion of Science have discovered a new mechanism for transporting and fixing specific proteins at specific sites of cell surfaces.
Some glycolipid species, called glycosylphosphatidylinositol (GPI), anchor specific proteins at adequate sites of the cell surfaces. Proteins anchored by GPI (GPI-anchored proteins) play a significant role in the signal transduction in the cell surfaces. Abnormality of the signal transduction is closely related to the carcinogenesis. In this work, we have discovered a new gene which synthesizes the lipid moiety of the GPI anchors, and found that when this gene becomes abnormal, the GPI-anchored proteins cannot exist in their specified primary sites of the cell surface.
We expect that, because this phenomenon may be related to the carcinogenesis mechanism, our finding will pave the way for early detection and curative technologies for cancer.
Details of this work were published in the electronic version of the American international journal, "Molecular Biology of the Cell" in October 4, 2006.
Proteins are synthesized in cells. Some proteins, such as digestive enzymes and constituents of blood, are secreted from the cells to function in extracellular space, while others are localized at membranes of the cell surface. These membrane proteins play important roles for signal transduction and energy exchange between inside and outside of the cell, and are closely related to the carcinogenesis and diseases. Such membrane proteins are one of the good targets for the development of new drugs. It is essential for proteins to adequately function so that they are properly transported to their specified primary sites and fixed there.
The GPI anchors are molecules that play a significant role in protein localization at plasma membranes. The anchors are a kind of glycolipid, and a portion of the membrane proteins is anchored at the plasma membranes via the GPI. The GPI-anchored proteins play an important role of signal transduction in the cell surface. The structures of the plasma membrane surface are not homogeneous, and therein domains with distinct characters, called the "microdomain" or "lipid raft," exist. It is shown that the GPI anchors are one of the members which constitute the microdomains.
Recently, it is reported that the GPI anchors are related to the carcinogenesis. For example, some reports have described that cancer is generated when enzymes which transfer GPI to proteins are abnormally produced. However, the mechanism of the carcinogenesis caused by the abnormality in the GPI synthesis pathway is as yet unclear.
AIST has investigated synthesis pathway of GPI-anchored proteins, and made a great contribution to the field of GPI synthesis using yeast cells as a model organism. We have recently clarified that a step of the inositol deacylation in the GPI-anchor synthesis pathway is closely related to the quality control of proteins in the ER. Furthermore, we have clarified that the GPI-anchored proteins are required for the recruitment of membrane proteins to the plasma membrane via microdomains.
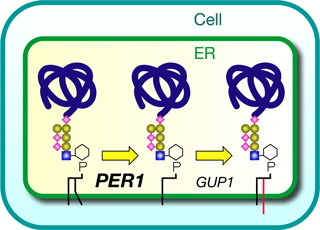
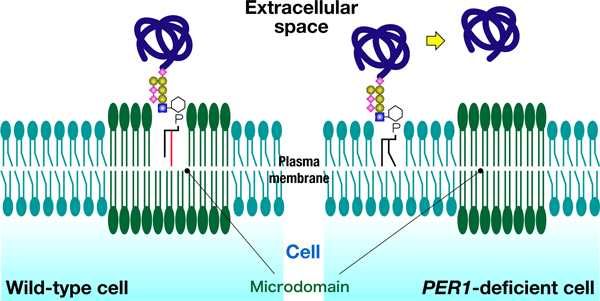
As shown in Figure 1, it is known as "lipid remodeling" that the initial and final lipid components of the GPI are different, but the mechanism of the lipid remodeling process and its physiological role have been unclear. Thus, we have tried to obtain genes related to the lipid remodeling pathway using budding yeast cells. As a result, we have obtained a new gene, PER1. From the investigation of PER1, we have clarified that PER1 is related to the removal of fatty acyl chain for replacing one of two fatty acyl chains. This is the first clarification of the initial process of the GPI remodeling. Moreover, we have found that when the removal of fatty acyl chain becomes abnormal, the GPI-anchored proteins cannot associate with the microdomains and are released to the outside of the cell (Figure 2). The physiological role of lipid remodeling in GPI synthesis has until now been unclear, but our work has clarified that the lipid remodeling is an essential mechanism for the recruitment of the GPI-anchored proteins in the microdomains.
 |
|
Figure 1. PER1 is involved in the removal of fatty acyl chain in the lipid remodeling process of GPI-anchored proteins. |
 |
|
Figure 2. Relationship between lipid remodeling and microdomains. In PER1-deficient cells, GPI-anchored proteins cannot associate with microdomains due to defect in lipid remodeling. |
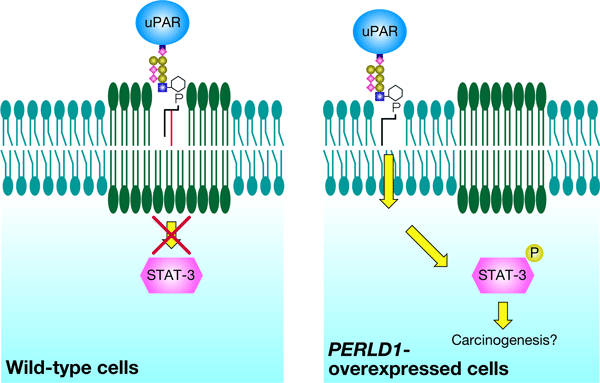
It is reported that the human PERLD1 gene is homologous to PER1, and unusual expression of PERLD1 is observed in breast and stomach cancer cells. However, the biological functions and the physiological role of PERLD1 are as yet unclear. We have clarified that the human PERLD1 gene has the same functions as those of the yeast PER1 gene, providing a new insight for understanding the mechanism of carcinogenesis. In other words, when the lipid remodeling becomes unusual by the abnormality of the PERLD1 gene, the GPI-anchored proteins cannot associate with the microdomains. We think that, since the microdomains work as the sites for signal transduction, the domains without the GPI-anchored proteins can violate the signal transduction, leading to the generation of cancer cells (Figure 3).
 |
|
Figure 3. A possible model for carcinogenesis in PERLD1-overexpressed cells. Unusual high activity of the PERLD1 gene product causes abnormal lipid remodeling of GPI-anchored proteins such as uPAR, and thus they cannot associate with the microdomains, resulting in excessive signal transduction. A protein STAT-3-P plays as a kind of switch controlling the expression of many genes. |
We expect that the findings in this work can be applied to the following:
-
By detecting abnormality of the lipid remodeling of human cells, the early diagnosis of cancer and the judgment of its stage can be carried out.
-
Utilizing activity assay systems of PER1 gene products, and thereby exploring chemical compounds inhibiting the activity, strong candidates for anti-cancer drugs can be screened.