- Cadmium-free bulk glass phosphors and thin film phosphors incorporating nanoparticles at high concentrations provide the impetus for applications in illuminations and displays -
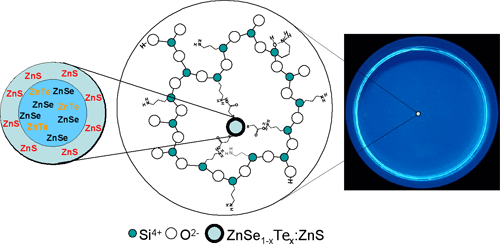
Semiconductor nanoparticles without using harmful cadmium have been prepared by an aqueous solution method at the National Institute of Advanced Industrial Science and Technology (AIST; Hiroyuki Yoshikawa, President). This study was supported in part by the Nanotechnology Glass Project, Nanomaterial Process Technology, Nanotechnology Program of the New Energy and Industrial Technology Development Organization (NEDO). The prepared luminescent nanoparticles show high emission efficiency of approximately 30%, with the emission peak wavelength in a blue-color region (450-460 nm). Furthermore, these nanoparticles were successfully incorporated in a glass matrix and exhibit stable emission (Figure 1, right).
Semiconductor nanoparticles have been expected to find their applications in various fields as new phosphors, but one obstacle is most of these semiconductor nanoparticles contain cadmium, which is harmful to the human body. On the other hand, ZnSe nanoparticles were known to exhibit emission peak in the ultraviolet region. In this report, we have succeeded in tuning the emission peak wavelength of ZnSe nanoparticles in to blue-color region by adding an appropriate amount of Te element to ZnSe nanoparticles and further forming core-shell structure by coating ZnS on the surface of nanoparticles (Figure 1, left).
Moreover, we have developed a novel and facile method (layer-by-layer self-assembly (LbL) method) for preparing glass thin-film phosphors utilizing self-organization effect, and succeeded in dispersing the nanoparticles in the films to extremely high concentration (0.01 mol/l). The brightness of the prepared thin films has been dramatically enhanced to up to 30 times of that of conventional phosphor (Y2O2S:Eu) at the same sample thickness. These prepared thin films are expected to be applicable in displays and illuminations as phosphors.
The details of this work had been presented at "nano tech 2006 International Nanotechnology Exhibition & Conference" held at Tokyo Big Sight on February 21-23.
[ Enlarged View ] |
|
Figure 1. Left: Illustrations of nanoparticles prepared by the addition of Te (inside) and S (outside) elements. Center: Illustrations of nanoparticles dispersed in glass matrix. Right: Blue luminescent images of the prepared glass irradiated with an ultraviolet light.
|
Phosphors have been widely used in illuminations and displays, which support our daily lives. Among the phosphors, dyes- and transition element ions- (transition metal ions and rare earth metal ions) dispersed inorganic materials have been used until now. Recently, it has been reported that semiconductor nanoparticles with 2-5 nm (one nm=10-9 m) in diameter exhibit high emission efficiency when the surface state of nanoparticles were controlled properly. Nanoparticles of CdSe and CdTe, which are compound semiconductors of elements in II and IV groups on the periodic table, have been extensively investigated. Group II-VI semiconductor nanoparticles also have features such as their size-dependent emission wavelength due to the quantum size-effect, and strong luminescence that is not easily saturated when increasing excitation light power because their emission decay times are short. Therefore, the nanoparticles are expected to be applied to various fields such as informational home electronics and biomarkers as new phosphors. However, the obstacle to application is these nanoparticles contain cadmium, which is harmful to the human body.
On the other hand, it is known that ZnSe nanoparticles, which also are group II-VI semiconductors, can exhibit luminescence at the ultraviolet wavelength region. However, ZnSe nanoparticles exhibiting emission at a wavelength region near 450 nm (which was brilliant blue for the human eyes) had not been obtained yet.
Since 2001, in the research project of NEDO mentioned above, AIST has carried out research into producing strongly luminescent materials by dispersing semiconductor nanoparticles in glass matrix.
In order to incorporate nanoparticles in glass, a sol-gel method starting from aqueous solutions was used. By improving the preparation conditions, we have obtained water-dispersible CdTe nanoparticles exhibiting emission efficiency which is approximately ten times higher than that of prior ones. Further, we have found a novel method for stably dispersing the nanoparticles in glass and have developed a kind of new "nano glass phosphors". However, recently, the use of cadmium has been strongly restricted in consideration of environmental problems. In particular, cadmium-free is essential concerning for the consumer products. Based on results of our previous studies, investigations were carried out aimed at the production of highly luminescent glass phosphors incorporating cadmium-free nanoparticles.
Strong emission is essential to put glass phosphors into practical use. However, the concentrations of nanoparticles in the reported glass are on the order of 10-5 mol/l. Therefore, higher concentrations of nanoparticles in glass are required for strong emission. Furthermore, it is necessary to compare the brightness of glass phosphors with that of conventional phosphors.
1) Synthesis of blue-emitting nanoparticles
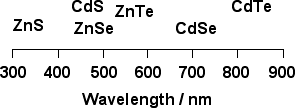
The emission wavelengths of group II-VI semiconductors in the bulk state are shown in Figure 2. There is a general rule that the emission wavelength increases with increasing atomic mass of the constituent atoms in the semiconductors. Although CdSe nanoparticles has been mainly used to get emissions in blue region, ZnTe nanoparticles is also a candidate material for blue emission.
 |
|
Figure 2. Emission wavelengths of group II-VI semiconductors (in the bulk state)
|
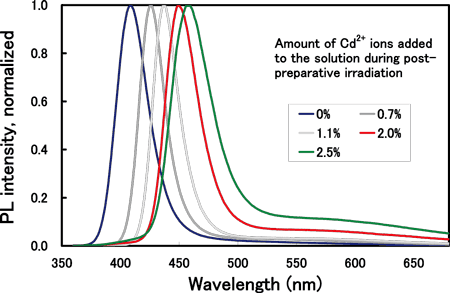
Therefore, we first tried to prepare ZnTe nanoparticles, but found that it is very difficult to determine the reaction conditions. In addition, there have been no reports on successful preparation of ZnTe nanoparticles. On the other hand, it has recently been reported that the emission efficiency of ZnSe nanoparticles can be enhanced by post-preparative irradiation. Furthermore, we have found that the emission wavelength can be increased by the addition of small amounts of Cd or Te to ZnSe. This phenomenon is consistent with the general rule shown in Figure 2, that is, an increase in the emission wavelength may be induced by the addition of heavy atoms. For example, as shown in Figure 3, by the addition of a small amount of Cd2+ ions into solutions during post-preparative irradiation of ZnSe nanoparticles, the emission peak wavelength can be shifted to around 450 nm in the blue color region.
In this work, we have succeeded in shifting the emission wavelength of ZnSe nanoparticles to a blue color wavelength of 450-460 nm by adding a small amount of Te2- ions, instead of Cd2+ ions, followed by covering the particle surface with ZnS. The emission efficiency of the nanoparticles we have produced is approximately 30%. Although this value is lower than that of the Cd-containing nanoparticles (approximately 50%), we have obtained bright luminescence for the Cd-free nanoparticles. As shown in Figure 1 left, the inner structure of the prepared nanoparticles does not contain any amount of Cd. Because it is difficult to keep the nanoparticles stable in the state of the solution, they were dispersed in glass to produce stable phosphors as shown in the center and right of Figure 1, using the method described above.
 |
|
Figure 3 Relationship between the emission peak wavelength and the amount of Cd2+ ions added to the solution during post-preparative irradiation of ZnSe nanoparticles
|
2) Development of a layer-by-layer self-assembly (LbL) method utilizing the self-organization effect for producing glass thin films incorporating nanoparticles at high concentrations
For bulk glass phosphors, aggregation of the nanoparticles can occur in the mixed solution when increasing the particle concentration. The upper limit of the concentration in the bulk glass was the order of approximately 10-5 mol/l, and thus more advanced methods were needed to increase the emission intensity.
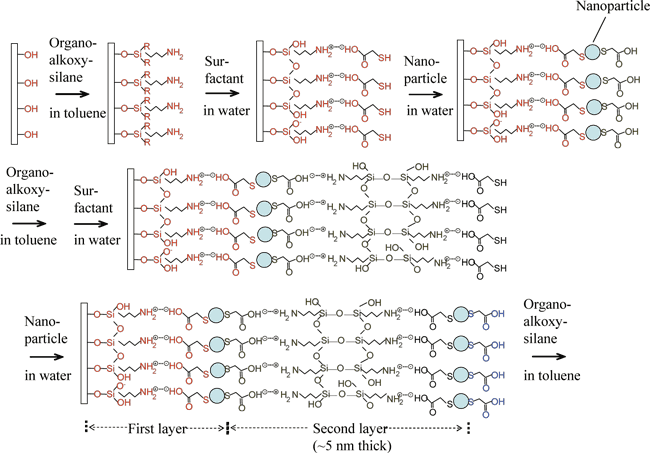
Based on the experimental results obtained in the preparation of bulk glass phosphors, as starting materials of glass, we have used organoalkoxysilane molecules with an amino (-NH2) group, which has good affinity to the surfactant on the surface of the nanoparticles. We have tried a method in which glass and nanoparticle layers are alternately stacked one layer by one layer on a substrate.
As a result, as shown in Figure 4, we have successfully prepared thin films in which nanoparticle layers and glass layers are alternately stacked. The film thickness was increased by 5 nm for each process. In this layer-by-layer self-assembly (LbL) method, because the mixing of sol-gel solution is not needed, the concentration of the nanoparticles can be greatly increased. The particle concentration in the films was increased up to 10-2 mol/l, when suitable preparation conditions were used. This particle concentration is very close to the concentration at which the luminescence intensity of the nanoparticles levels off due to the transfer of excitation energy between the particles, thus indicating that we can achieve a high particle concentration close to the upper limit. The emission efficiency of the thin films dispersing nanoparticles is approximately 24%, exhibiting bright luminescence. Also, by changing the affinity of the nanoparticles to the substrate, e.g., by painting water-repellent inks on the substrate, we can coat only the desired places on the substrate with the nanoparticles, enabling the formation of fine patterns using the nanoparticles.
 |
|
Figure 4 Formation of a glass thin film dispersing nanoparticles at high concentration by the LbL method utilizing self-organization effect
|
3) Comparison of performance
Due to a restriction of excitation wavelength, we evaluated the brightness of red-emitting LbL glass thin film (thickness: 39 nm) incorporating CdTe nanoparticles having nearly the same emission efficiency of the blue-emitting ZnSe nanoparticles, and compared with the conventional phosphor Y2O2S:Eu (P22-RE3, produced by Kasei Optonix Ltd.). This phosphor (Y2O2S:Eu) is a typical red-emitting one which is promising for the production of white LEDs. The mean diameter of the particles in the phosphor is several micrometers, which were molded in the film of 0.1 mm thick to detect the brightness. The brightness for 39 nm thickness was obtained using optical filters. The result is shown in Figure 5.
 |
|
Figure 5 Comparison of brightness of a LbL glass thin film incorporating nanoparticles (a) with that of a conventional phosphor (b) of the same sample thickness, using the same excitation light source
|
As shown in this figure, the LbL glass thin films can exhibit higher brightness than the conventional phosphor at the same sample thickness. For example, when both materials are excited by an ultraviolet light of 365 nm, the brightness of the former is estimated to be 30 times higher that of the latter. Thus, it can be considered that the glass thin films dispersing nanoparticles are suitable for generating strong fluorescence from thin and narrow regions. In the near future, when the power of the LED is further increased, our developed thin film phosphors can be expected to be used for illuminations and displays.