National Institute for Advanced Industrial Science and Technology (AIST, Hiroyuki Yoshikawa, President) has succeeded in the development of an efficient method to decompose environmentally persistent and bioaccumulative perfluorooctane sulfonate (PFOS) and related compounds to fluoride ions.
Organofluorine compounds have been widely used in many industries as surfactants because they have excellent properties such as heat and chemical resistance, light transparency, etc. However, some of them show high environmental persistence and bioaccumulation so that the development of effective waste treatment methods is desired. PFOS has been globally detected in environmental waters and wildlife and its long-term toxicity (negative health effects caused by continued intake of the substance) is suspected. PFOS shows very high chemical and thermal stability; it cannot be decomposed even if it is boiled in sulfuric acid.
AIST has achieved highly efficient decomposition of PFOS into fluoride ions by adding iron powder to water containing PFOS and taking the sample to subcritical water state at 250-350°C. The produced fluoride ions may be recycled as a source of fluorine using an established processing method for fluoride ions. This method was successfully applied to decompose other related fluorochemicals and to decompose PFOS contained in a coating agent used in electronic industry.
Details of this method were published in the February issue (No. 3) of Environmental Science & Technology, Vol. 40, published by the American Chemical Society.
Organofluorine compounds have unique characteristics (repulsion to water and oil, resistance to heat and to chemical substances, non-absorption of light) so that they have been used as surfactants such as surface treatment agents, emulsifiers, coatings, etc. However, it has been recently reported that some of them persist in the environment and accumulate in wildlife. PFOS is a representative example. Because of the environmental persistence and bioaccumulation of PFOS, the United States Environmental Protection Agency (USEPA) established regulations controlling the use of PFOS in April 2002, and in November of the same year, the Organization for Economic Cooperation and Development (OECD) published a hazard assessment of PFOS. In December of the same year, PFOS became a designated compound according to the Chemical Substances Control Law (currently class II specified chemical substance) in Japan. In June of 2005, PFOS was nominated at the Stockholm Convention and its international regulation is being examined.
Therefore, it is extensively desired to develop effective waste treatment techniques for PFOS and related compounds with low energy costs.
AIST has been conducting research on the analytical methods, environmental fate, and decomposition methods for organofluorine compounds.
The outline of this decomposition method is as follows. A stainless steel reactor (volume 34.3 mL) containing an aqueous solution of PFOS (10 mL, PFOS concentration: 46-186 ppm) and iron powder (0.54 g) or other metal powders, was introduced in an argon atmosphere. Then, the temperature was raised to the subcritical state at 250-350°C.
After a certain time, the reactor was cooled to room temperature and the components in the reactor were analyzed. A reaction without metal powder was also performed. When no metal powder was added, little PFOS was decomposed. The highest decomposition of PFOS was achieved when iron powder was used.
For example, when an initial PFOS concentration was 186 ppm and the reaction temperature was 350°C [pressure in the reactor was 23.3 MPa (1 MPa = 9.87 atmospheric pressure)], PFOS completely disappeared in water after six hours of the treatment, and at the same time, fluoride ions efficiently produced. The PFOS decomposition occurred on the iron surface. After six hours of the treatment, organofluorine compounds (PFOS or its reaction intermediates) were still detected on the surface of iron. However, after prolonged treatment, all organofluorine species on the surface disappeared.
This method was also effective to the decomposition of shorter-chain related fluorochemicals (perfluoroaklyl sulfonates) and was successfully applied to the decomposition of PFOS contained in an a coating agent used in electronic industry.
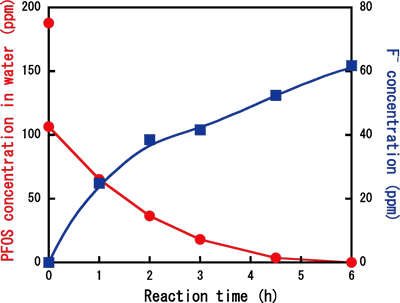 |
Figure
Decrease in PFOS concentration and increase in fluoride-ion concentration by subcritical water treatment with iron powder. Initial concentration of PFOS: 186 ppm, Reaction temperature: 350°C, Reaction pressure: 23.3 MPa [Reprinted with permission from Environmental Science & Technology, 2006, 40, 1049-1054. Copyright 2006. American Chemical Society]. |