- Toward the creation of new curative medicines for candidiasis etc. -
The National Institute of Advanced Industrial Science and Technology (AIST, President: Hiroyuki Yoshikawa) and the University of Tsukuba (President: Yoichi Iwasaki) found a new mechanism to exclude abnormal proteins by designing an unusual protein which is not adequately folded in the cell.
A group of proteins with glycolipids, called "GPI anchors," exist in cells. In this work, we have found a new quality-control mechanism for these proteins using an artificially designed abnormal protein in cell.
This finding may contribute to the development of new curative medicines for fungal infections, e.g., candidiasis. Moreover, this can be expected to clarify the onset mechanisms of incurable diseases such as prion disease and hypophosphatasia, leading to the development of medical treatments.
The details of this work were published in the electronic edition of the US international journal "Molecular Biology of the Cell" on November 30, 2005.
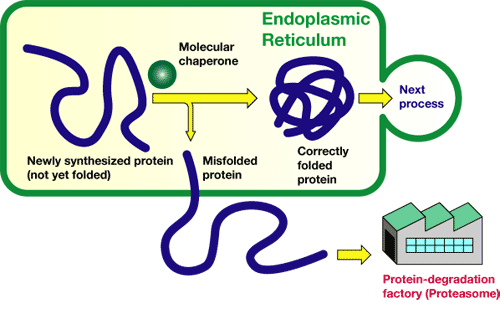
Proteins play important roles in the human body. Muscles consist of proteins, as do enzymes, which regulate biological reactions. A part of the proteins is synthesized in a small cavity (organelle), called the "endoplasmic reticulum." Newly synthesized proteins have a string-like structure, and the strings must be adequately folded sterically so that the resultant proteins work normally.
For this purpose, molecules called "molecular chaperons" exist in the endoplasmic reticula to help sterically adequate folding of the protein strings. However, proteins are sometimes not adequately folded even with the molecular chaperon's help. Such unusual proteins are excluded from the endoplasmic reticula and decomposed in a protein-degradation factory called "proteasome." That is, the endoplasmic reticulum possesses a function to distinguish inadequately folded proteins from folded ones in order to dispose of the unusual proteins.
This functional mechanism is referred to as the "quality-control mechanism of proteins in the endoplasmic reticulum." If this mechanism is broken, cells cannot judge which proteins are adequate "products," inducing severe, life-threatening damage. For membrane proteins and water-soluble proteins, the quality-control mechanism in the endoplasmic reticulum has already been discovered, but many events cannot be explained by their quality-control mechanisms.
 |
|
Figure 1 A quality-control mechanism for proteins in endoplasmic reticulum
|
Proteins are synthesized by the binding of amino acids, but this alone does not complete them. In some cases, only when some linked amino acids are modified with sugar chains they become functional proteins. One of the modifications is the addition of glycolipids called "GPI anchors."
GPI-anchored proteins are known to be related to various diseases. The prion protein, which induce bovine spongiform encephalopathy (BSE), is one example, and recently human hypophosphatasia has been suggested to be due to an abnormality of a GPI-anchored protein. The GPI-anchored proteins also exist on the cell surface layer of Candida yeast and malaria plasmodium, which invade the human body, and trypanosome protozoa, which induces sleeping disease, and they play an important role in the human pathogenic onset.
Also, research works regarding the synthesizing mechanisms of the GPI-anchored proteins are important in order to clarify the causes of various diseases. If the synthesizing process of the GPI-anchored proteins is fully understood, development of anti-mycotic (e.g., for candidiasis), anti-malarial and anti-protozoal drugs can be expected by searching for chemical compounds which target the process.
Considering the above-mentioned facts, the Research Center for Glycoscience of AIST has investigated the synthesizing mechanism of the GPI-anchored proteins, and is one of the most worldwide eminent laboratories in this research field using yeast cells.
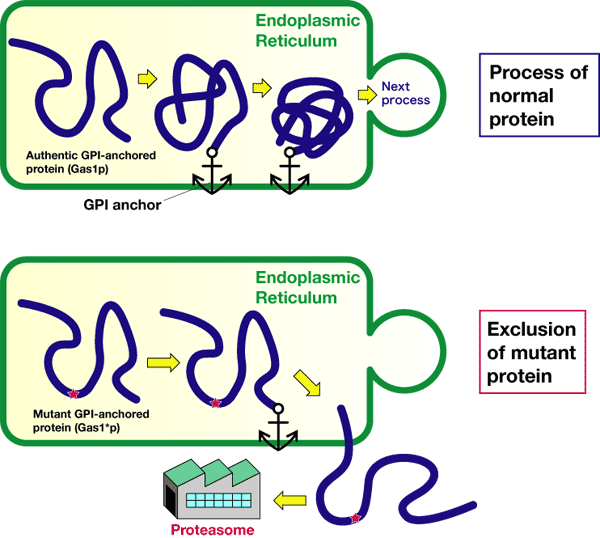
Until now, it has been unclear where, when, and how the quality-control of GPI-anchored proteins is carried out. Thus, to aim at the clarification of the quality-control mechanism of GPI-anchored proteins, we have induced a GPI-anchored protein which is not adequately folded in the endoplasmic reticulum, by introducing an artificial mutation in budding yeast systems.
We have examined where and how this abnormal protein is degraded depending on time. Using proteasome inhibitors and mutants, we have found that when the protein is not adequately folded, it is excluded from the endoplasmic reticulum to be decomposed and then removed by proteasome, as shown in Figure 2. We have also found that this degradation system is a third quality-control mechanism different from those for systems of membrane proteins and water-soluble proteins
 |
|
Figure 2 Process of an abnormal protein (mutant GPI-anchored protein Gas1*p) generated by AIST.
|
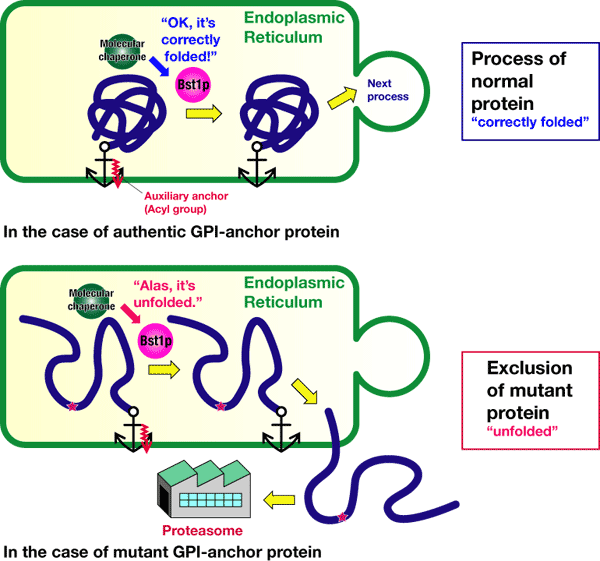
Using inhibitors and mutants, we have examined how normal and abnormal proteins are distinguished. As shown in Figure 3, removing a part of the GPI anchor or auxiliary anchor (acyl group, indicated in red) is necessary for the GPI-anchored protein to proceed to the next step from the endoplasmic reticulum. This auxiliary anchor can be removed by an enzyme called Bst1p. If the auxiliary anchors are removed, both normal and abnormal proteins can proceed to the next step. We have clarified that, after receiving information regarding the protein folding from a "molecular chaperon," Bst1p decides whether the GPI-anchored proteins go to the next step or are excluded from the endoplasmic reticulum to be decomposed and removed. In other words, Bst1p plays the role of having final responsibility for the quality-control of the GPI-anchored proteins in the endoplasmic reticulum.
 |
|
Figure 3 An administrator for the quality-control of GPI-anchored proteins, Bst1p, determines the destination of the proteins after receiving information that "correctly foleded" or 2misfolded."
|