The Research Institute for Ubiquitous Energy Devices (RIUED) of the National Institute of Advanced Industrial Science and Technology (AIST) has developed ionic liquid electrolyte consisting of asymmetric cyclic quaternary ammonium-imide salt, as flame-resistant electrolyte for ensuring safety of lithium metal secondary battery. which promises a drastic increase in energy density.
While the lithium metal secondary battery is expected to provide twice or more as high energy density as existing lithium ion secondary battery, its practical application has been hampered by two impeding factors: (1) lithium anode forms dendritic deposits in organic electrolyte solution to cause inside short-circuit, and (2) coexistence of chemically active lithium metal with inflammable organic solvent makes it difficult to secure safety in commercial use. For ensuring safety of the lithium metal secondary battery, flame-resistant electrolyte is needed such as ionic liquid, but no ionic liquid, which is stable in the voltage region of lithium battery, has been available.
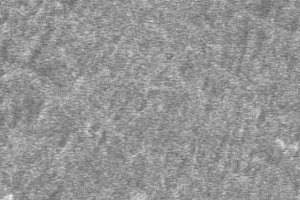
The newly developed ionic liquid electrolyte made of cyclic quaternary ammonium-imide salt is stable in wide voltage range of the lithium battery, and provides charge-discharge efficiency of lithium metal electrode which is equivalent to that in organic liquid electrolyte. Lithium deposition on a lithium metal substrate after deposition/dissolution cycles in a test cell did not form the dendritic structures but in flat and smooth form.
 (a)
(a)
|
(b)
|
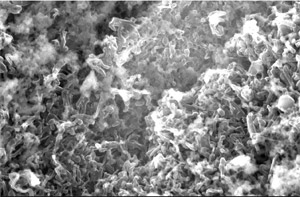
Figure 1 Morphology of the surface of Li electrode after the deposition/dissolution of Li more than 100 cycles in (a) the ionic liquid developed in the AIST and (b) the conventional organic electrolyte.
|
This ionic liquid is thermally stable, flame-resistant, non-volatile and has high lithium charge-discharge efficiency, opening the way to the practical use of lithium metal secondary battery. Furthermore, the use of ionic liquid is expected to make portable electronic devices lighter and their service life longer.
The R&D work has been supported by a contract project “R&D on Li Batteries for FCV and HEV (FY02~06)” entrusted by the New Energy and Industrial Technology Development Organization (NEDO).
Further R&D efforts will be focused on the optimization of purity and composition of the ionic liquid and upgrading of lithium metal electrode efficiency.
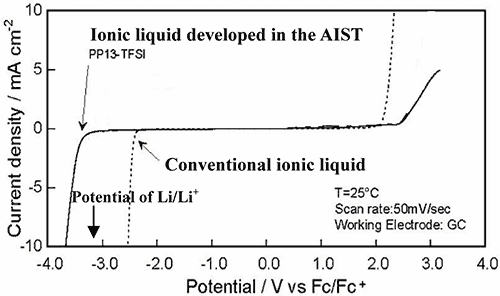
The lithium metal secondary battery can hold energy density twice or more as high as that of the existing lithium ion secondary battery, and expected to contribute to drastic upgrading of user- friendliness of compact portable electronic devices. However, organic electrolyte solution developed for lithium metal secondary battery has two drawbacks: dendritic deposition of lithium on anode leads to inside short-circuit, and the coexistence of chemically active lithium metal with combustible organic solvent, making it difficult to secure safety in the practical application. As the lithium metal secondary battery can be only storage battery promising significant upgrading of energy density, much efforts have been paid to search of flame-resistant electrolyte to secure adequate safety. While ionic liquid has been regarded as the most hopeful electrolyte of being flame-resistant, there has been no ionic liquid which withstands the voltage of lithium battery, in particular, the voltage at the lithium metal anode, as illustrated by the potential window for existing ionic liquid in Fig. 2.
 |
|
Figure 2 The comparison of the electrochemical window (the available potential region) of the conventional ionic liquid and PP13-TFSI in this development.
|
The RIUED-AIST has possessed since earlier research potential for ionic liquid, which was named formerly as room temperature molten salt, leading the world, and tried to develop various electrochemical devices. Recently, the study has borne fruits in the form of ionic liquid consisting of various asymmetric quaternary ammonium-imide salt which is stable in the operating voltage range of lithium metal secondary battery, and led to the full-scale verification of its applicability of to lithium secondary battery under the NEDO-entrusted project “R&D on Li Batteries for FCV and HEV (FY02~06)” since fiscal 2002.
Conventional ionic liquids in the past studies are not fully applicable to lithium battery because of high reactivity with lithium. The newly developed ionic liquid electrolyte consists of asymmetric cyclic quaternary ammonium-imide salt, N-methyl-N-propylpiperidinium bis(trifluoromethane sulfonyl) imide (PP13-TFSI), of which molecular structure is shown in Fig. 3, and is found to be stable in the operating voltage range of lithium battery. The charge-discharge efficiency has proved to be 97 % or higher with coin-type lithium/lithium cobaltate battery. The charge-discharge efficiency of lithium metal in PP13-TFSI with nickel substrate has proved to be 97 %, against 98 % in organic electrolyte solution. The deposited lithium on lithium metal substrate after repeated cycles of deposition/dissolution in the test cells does not form usual dendritic shape, but of relatively flat and smooth geometry.
The newly developed ionic liquid has little weight reduction up to around 300°C due to combustion or pyrolysis in the course of heating up, is excellent in thermal stability, flame resistance and non-volatility. Besides, its charge-discharge efficiency is adequately high and the lithium deposition is of good shape, to be helpful for the practical application of lithium metal secondary battery in respect both safety and performance. The battery will contribute to further weight reduction and life extension of portable electronic devices.
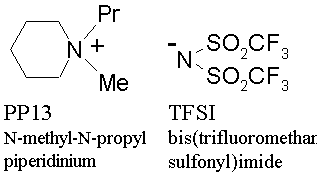 |
|
Figure 3 The structure of the ionic liquid of asymmetric quaternary ammonium-imide developed in AIST |