Update(MM/DD/YYYY):12/03/2004
Cost Reduction for Secondary Lithium-Ion Battery
- New Lithium Manganese Oxide Cathode Materials with High Voltage and Capacity Developed -
Key Points
-
There has been available no manganese oxide cathode materials for secondary lithium-ion battery comparable to existing cathode materials of lithium cobalt oxide in respect to both of discharge voltage and capacity.
-
New lithium manganese oxide cathode materials has been prepared through the ion exchange synthesis process in low-temperature molten salt of sodium compound, achieving mean discharge voltage 3.61V and initial discharge capacity 168mAh/g.
-
Replacing manganese partly with titanium may upgrade the capacity further to 177mAh/g.
-
Utilization of richly available and low-cost resources such as manganese oxide and titanium oxide makes it possible to reduce cathode materials price to about 1/5, and that of secondary lithium-ion battery by around 30%.
Synopsis
The Advanced Manufacturing Research Institute (AMRI) of the National Institute of Advanced Industrial Science and Technology (AIST), an independent administrative institution, has developed new cathode materials for secondary lithium-ion battery, based on lithium manganese oxide prepared through the ion exchange synthesis in low temperature molten salt using sodium compound, in collaboration with the Research Institute for Ubiquitous Energy Devices (RIUED), AIST. The cathode materials plays a decisive role in determining the performance of lithium secondary battery, and the newly developed materials has initial discharge current capacity 168mAh/g and discharge energy density 606mWh/g, which is comparable to most widely used materials, lithium cobalt oxide with 160mAh/g and 630mWh/g, respectively. Partial replacement of manganese with titanium upgrades the performance further to current capacity 177mAh/g and energy density 635mWh/g.
The use of secondary lithium-ion battery has been has been rapidly expanded in these days as power sources for various kinds of personal digital assistance (PDA), and is expected to be further spread into larger-sized applications such as fuel cell-driven and hybrid automobiles. While cathode materials for secondary lithium-ion battery has been so far made mostly from lithium cobalt oxide, the development of substitute materials jab been needed because of depletion in and soaring prices of cobalt resources.
The AMRI/ RIUED-AIST has been engaged in R&D works of new cathode materials by applying low temperature synthetic process, one of elevated efficiency manufacturing technologies, as a part of contract project (FY2002~2006) sponsored by the Ministry of Economy, Trade and Industry (METI) and the New Energy and Industrial Technology Development Organization (NEDO).
The newly developed lithium manganese oxide cathode material has discharge voltage as high as 4V, which is highest among that of manganese oxide-based compounds, and 0.3V higher than that of existing lithium cobalt oxide materials. The achievements may be attributed to keeping the original crystal structure of sodium compound as starting template and preventing residual sodium from blocking lithium ion insertion/extraction, through the optimization of conditions for ion exchange synthetic process in low temperature molten salt. The application of the materials to secondary lithium-ion battery is expected to reduce the price of cathode materials to about 1/5, and that of battery by around 30%.
The subsequent efforts will be focused on further upgrading of charge-and-discharge characteristics and increasing capacity through the optimization of grain size control and chemical composition, and on establishing low cost manufacturing process. The results of the present study will be reported at the 45th Battery Symposium in Japan to be held November 27 to 29, 2004 , at Kyoto International Conference Hall.
Background
The secondary lithium-ion batteries are characterized by small size and high output voltage, and currently mounted in most of personal digital assistances (PDAs) as power sources, such as mobile phones and notebook PCs. The significance of the batteries will be further extended to practical applications to large-sized power supplies for automobile use. In the secondary lithium-ion battery, the action of charge/discharge is done through exchange of lithium ions between cathode and anode, of which the cathode material is more important for deciding the battery performance. The discharge capacity depend upon how much lithium ion can be taken in and provided (insertion-extraction), while discharge voltage is defined by materials and its crystal structures. For this reason, studies have been focused so far on lithium-containing transition metal oxide, such as lithium cobalt oxide (LiCoO2) and lithium nickel oxide (LiNiO2) with layered rock-salt structure, and lithium manganese spinel (LiMn2O4) with spinel structure. In most of the existing secondary lithium-ion batteries, lithium cobalt oxide has been used for cathode materials. In view of recent rise in cobalt price and requirements for cost reduction, however, it has been strongly desired to develop new cathode materials based on richly available and low-priced manganese.
While the most promising candidate for cathode materials is lithium manganese spinel, its discharge capacity is 120mAh/g, much smaller than that of lithium cobalt oxide (160mAh/g), and the need for developing other substitute materials is still urgent. Above all, much interest has been focused on high capacity lithium manganese oxide starting from the alkali manganese oxide with one-dimensional tunnel structure. However, it is often difficult to replace different alkali ions contained in starting materials fully with lithium ion, and all of these substitutes have discharge voltage about 1 V lower than that of lithium cobalt oxide, to be unsuited for the replacement.
History of R&D Work
The AMRI-AIST has been engaged, in collaboration with the RIUED-AIST, in a project (FY2002~2006) sponsored by the Ministry of Economy, Trade and Industry (METI) and the New Energy and Industrial Technology Development Organization (NEDO). In this project, one of high efficiency manufacturing technologies, low temperature manufacturing process, has been applied to the preparation of battery materials, aiming at new lithium manganese oxide cathode materials of comparable performance as that of lithium cobalt oxide.
The low temperature manufacturing process is an important technology for reducing cost of materials preparing process, and at the same time, useful for design and development of new functional ceramics. The present success was achieved through the development of materials synthesis technology for raising the discharge voltage of new lithium manganese oxide Li0.44MnO2 with tunnel structure. This compound was known to have discharge voltage as low as 3V or so, but to be discharged and recharged very quickly.
As the cathode materials is made from oxide compounds mainly composed of richly available and low-priced manganese and titanium, the price of raw oxide materials is about 1/5 that of existing lithium cobalt oxide materials, and the price of lithium secondary battery is expected to be reduced to around 30%.
Details of R&D Work
The success in raising discharge voltage of newly developed lithium manganese oxide Li0.44MnO2 has been achieved by extensively reducing residual sodium in the starting materials which prevents insertion/extraction of lithium ion. The synthesis takes place in the following steps: (1) sodium carbonate is heated with manganese dioxide at 900 °C to make sodium manganese oxide Na0.44MnO2 with targeted tunnel structure. (2) The resultant black powder is processed in low temperature molten salt prepared by heating a mixture of lithium nitrate with lithium chloride in air at 280 to 330 °C, to replace sodium with lithium while keeping the tunnel structure. (3) In this process, heating at temperatures lower than 280 °C leaves sodium in starting materials without being replaced with lithium. (4) On the other hand, heating at temperatures higher than 330 °C, the tunnel structure may be broken down. (5) In this way, optimizing the ion exchange conditions in molten salt has made it possible to reduce the Na/Li ratio to less than 0.01, which is 1/5 of earlier ratio.
For the purpose of revealing discharge voltage of new cathode materials and clarifying the potential of upgrading capacity, a coin-type lithium battery has been manufactured with the “black powder” as cathode and metal lithium as anode (Fig. 1), and its initial discharge characteristics has been measured for a voltage range 2.5~4.8 V, current density 5mA/g and at temperature 30 °C. The resultant average discharge voltage was 3.61 V, and discharge capacity 168mAh/g (Fig. 2). The operating voltage made available through the present synthetic process is 0.3 V higher than that of the existing lithium cobalt oxide and the highest among manganese oxide-based cathode materials.The newly developed cathode materials opens the way to upgrading discharge voltage and capacity, and its application is expected to be extended to in-vehicle battery for fuel cell-driven and hybrid automobiles which require larger size and higher performance.
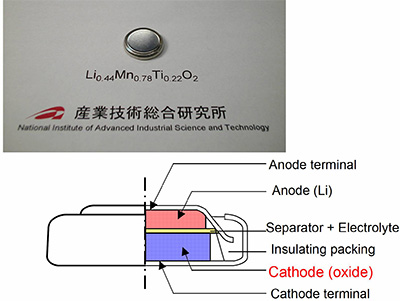 |
Fig. 1. Prototype coin-type lithium battery and its construction
A prototype coin-type battery (#2032 cell) with titanium-substitute of the newly developed lithium manganese oxide (Li0.44Mn0.78Ti0.22O2) as cathode materials. |
|
|
|
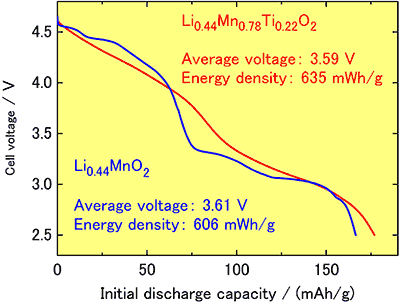 |
Fig. 2. Initial discharge curves of newly developed lithium manganese oxide cathode materials and its capacity upgraded by substitution with titanium, using metal lithium for anode after charging up to 4.8 V. |
Additionally, it has been found that the capacity can be further upgraded by substituting manganese partly with titanium. Particularly, with 22 % substitution of manganese with titanium, (Li0.44Mn0.78Ti0.22O2) shown in Fig. 3, the initial discharge capacity is shown to be as high as 177 mAh/g (Fig. 2). Even after 10 cycles of charge-discharge, the capacity remains 167 mAh/g, presenting excellent cycle characteristics.
The newly developed cathode materials is compared with the existing materials, lithium cobalt oxide and lithium manganese spinel, in respect to discharge capacity and energy density (Fig. 4).
 |
Fig. 3. Black powder of newly developed titanium substitute of lithium manganese oxide (Li0.44Mn0.78Ti0.22O2). |
|
|
|
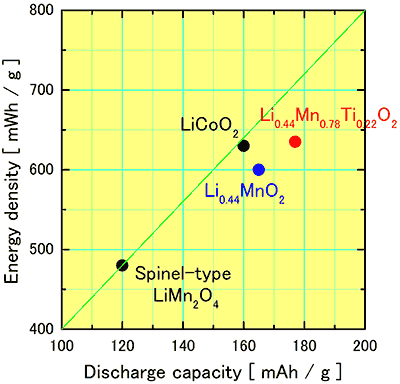 |
Fig. 4. Compared performance of 4 V-class cathode materials
The newly developed lithium manganese oxide Li0.44MnO2 and its titanium substitute Li0.44Mn0.78Ti0.22O2 have potential performance far exceeding that of lithium manganese spinel LiMn2O4 and comparable to that of lithium cobalt oxide LiCoO2. |