- World First Success in Large-Scale Computational Simulation of Chemical Reactions in Supercritical Water -
The Research Institute for Computational Sciences (RICS) and the Supercritical Fluid Research Center (SFRC) of the National Institute of Advanced Industrial Science and Technology (AIST), one of independent administrative institutions, have successfully opened the way to understanding the mechanism of peculiar reactions taking place in supercritical water in collaboration with the Graduate School of Pure and Applied Sciences and the Special Research Project on Nano-Science of the University of Tsukuba (UOT); and the Department of Chemistry and Applied Biochemistry (DCAB) and the Computational Science Group, USI-Lugano of the Eidgenössische Technische Hochschule Zürich (ETHZ). One of reactions in question refers to the Beckmann rearrangement where e-caprolactam is produced from cyclohexanone oxime in supercritical water without using concentrated sulfuric acid. It has been successfully demonstrated, first in the world by the computer simulation based on the first principle molecular dynamics, that the rearrangement is made through incomplete hydrogen bond network created owing to peculiar density property of supercritical water, neither liquid nor gas, but an intermediate state between them. Such a reaction mechanism is expected to be applicable to other processes based on peculiar reactions experimentally found to occur in supercritical water without additives such as strong acid or alkali.
It was originally demonstrated by the SFRC-AIST that the industrial synthesis of e-caprolactam, a monomer of nylon, through Beckmann rearrangement of cyclohexanone oxime in the presence of concentrated sulfuric acid could take place in supercritical water without strong acid. It was difficult, however, to identify the optimum conditions for the production by this method, because of uncertainties in the reaction mechanism.
As a result of the computer simulation based on the first principle molecular dynamics, it is revealed that in water of normal density (1g/cm3) and temperature 400°C, a hydrogen ion is surrounded by water molecules forming stable and complete hydrogen bond networks and no reaction takes place to make e-caprolactam. On the other hand, in supercritical water of 0.7g/cm3 density and temperature 400°C, the hydrogen bond network fails to fully grow and a hydrogen ion is surrounded by smaller number of water molecules. Consequently, hydrogen ions turn more active to rearrange cyclohexanone oxime into e-caprolactam. It is shown, therefore, that the production of e-caprolactam in supercritical water is attributable to incomplete hydrogen bond networks caused by peculiar density properties of supercritical water.
The present computer simulation work has made it feasible to replace the industrial synthesis of e-caprolactam using concentrated sulfuric acid with that in supercritical water. Moreover, the first principle molecular dynamics proved to be useful for predicting various chemical properties under supercritical conditions without making risky experiments at high temperatures and pressures otherwise needed.
* The result of this work will be published in the Journal of The American Chemical Society, June 2, 2004 and on-line at the website of this Journal, April 28.
For the industrial production of e-caprolactam, a monomer of nylon, the Beckmann rearrangement with concentrated sulfuric acid is usually resorted to. In this process, however, a great amount of ammonium sulfate is released as by-product. While ammonium sulfate itself is not hazardous, disposing it may pose a problem. Besides, owing to the use of high temperature concentrated sulfuric acid, the process involves needs for reducing environmental burden and ensuring safety, it has been urgently sought for to establish a synthetic method of e-caprolactam without using sulfuric acid.
Dr. Yutaka Ikushima, Deputy Director of SFRC-AIST and his colleagues found that the Beckmann rearrangement occurred in supercritical water to produce e-caprolactam from cyclohexanone oxime without using concentrated sulfuric acid.
However, as the mechanism of this reaction was not adequately known at that time, it was rather difficult to identify the optimum conditions for production and to explore new reactions. While the supercritical state of high temperatures and pressures is hardly realizable experimentally, the reaction can be effectively examined through the computer simulation, if the enormous machine time is accepted. For this reason, the study on the mechanism of supercritical reactions has been initiated through the computer simulation.
(1) Hydrogen Bond Network and Reactivity
The computer simulation was carried out through the first principle molecular dynamics (Car-Parrinello method) which allows analyzing the interaction of target molecules with water molecules at a time resolution of picosecond (1ps =10-12s). As this method requires a great computer resource, the following situation was assumed: a molecule of cyclohexanone oxime and a hydrogen ion are put into a unit cell containing 60 water molecules. The reaction was simulated for a few ps on the ORIGIN computer of Japan Silicon Graphics Inc. (SGI) involving 40CPUs taking a few weeks. The simulation for 1ps takes about 3 days.
The computer simulation revealed that the hydrogen bond network is not fully developed in supercritical water (temperatures 400°C, density 0.3~0.7g/cm3). In another state of supercritical water (temperatures 400°C, density 1g/cm3, let us designate this state as “quasi-supercritical” for the sake of convenience), every hydrogen ion is fully surrounded by water molecules to be stabilized by complete hydrogen bond networks (Fig.1b), and the reaction leading to the synthesis of e-caprolactam fails to occur. On the other hand, in supercritical water (temperatures 400°C, density 0.7g/cm3), the hydrogen bond network is underdeveloped with every hydrogen ion surrounded by a reduced number of water molecules (Fig.1a). Consequently, activated hydrogen ion reacts with cyclohexanone to produce e-caprolactam, playing the role of active hydrogen ion provided by concentrated sulfuric acid in the conventional process.
Actually, the simulation under the conditions of temperatures 400°C and density 0.7g/cm3 shows that cyclohexanone oxime reacts with hydrogen ion within 1ps and ultimately turns into e-caprolactam via an intermediate body (Fig.2a). With conditions of temperature 400°C and density 0.7g/cm3, however, no reaction occurred in 3.4ps (Fig.2b). Though the conditions of temperature 400°C, and density 1.0g/cm3 are hardly realizable experimentally because of high pressure required, it has been shown by the experiment that e-caprolactam is not formed under the the quasi-supercritical state with slightly lower temperatures and pressures and density close to 1.0 g/cm3. (In practice, cyclohexanone oxime is hydrolyzed into cyclohexanone.)
(2) Reaction Selectivity
Another cause for different reactions in supercritical and quasi-supercritical water has been identified. In the normal liquid water of density 1.0g/cm3, every molecule is fully surrounded by water molecules. In supercritical water where the density is so low that it fluctuates, a “dry area” is left without water molecules. The fluctuation or inhomogeneity of density is a characteristic feature of the supercritical fluid. Nitrogen atom of cyclohexanone oxime is “dry” in supercritical water and rejects hydrogen ion which moves along chain of water molecules. On the contrary, oxygen atom is “wet” to react with hydrogen ion ultimately to form e-caprolactam. In quasi-supercritical water of density 1.0g/cm3, every molecule is “wet” to be fully surrounded by water molecules, and hydrogen ion interacts with nitrogen atom of cyclohexanone oxime. As a result, cyclohexanone oxime is hydrolyzed in quasi-supercritical water to return to cyclohexanone. Oxygen atom is also “wet” to allow hydrogen ion accessing, but no e-caprolactam is formed because of hydrogen ion exchange reaction.
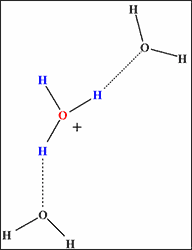
a) In supercritical water
(Incomplete hydrogen bond network)
|
|
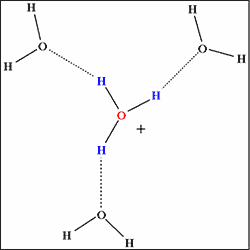
b) In liquid water
(Complete hydrogen bond network)
|
|
Fig. 1 Hydrogen bond network surrounding hydrogen ion (its hydrated form H3O+). |
(3) Needs for Supercritical State
The density of supercritical water around 0.3~0.7g/cm3 is of great significance. At temperatures lower than the critical point, the supercritical state is not available as the system is separated to liquid water of 1.0g/cm3 and vapor. In order that the Beckmann rearrangement proceeds in water, the supercritical state is essential, where molecules are not distributed uniformly, but dispersed in the form of clusters. As the clusters fluctuate with time, the entire system consists of ever changing mosaic structures of stable and unstable hydrogen ion areas. This state is a “must” for reactions in supercritical water.
(4) Summary of Reactions
In supercritical water, hydrogen bond network is not developed well because of lower density and hydrogen ion with enhanced activity reacts with oxygen atom in cyclohexanone oxime to form intermediate compound of a positive charge, which ultimately turns into e-caprolactam through the migration of hydrogen via a chain of water molecules. In quasi-supercritical water with higher density, hydrogen ions are stabilized by complete hydrogen bond network, and intial cyclohexanone oximeis hydrolyzed into cyclohexanone through the interaction with nitrogen as in case of usual acid-base reactions.
|
|
|
Fig. 3 Summary of reactions
• Path 1 (reactions in supercritical water): hydrogen ion gets access to “wet” oxygen atom, O, surrounded by water molecules causing reaction, while “dry” nitrogen atom, N, is not involved in the reaction.
• Path 2 (reactions in quasi-supercritical water): both O and N are “wet” and stable hydrogen ion reacts with N. (O does not react with stable hydrogen ion.)
|