- Breakthrough for the research on protonics: future technology using protonic phenomena -
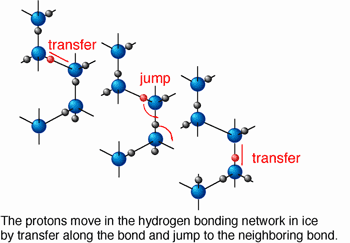
The protonic diffusion in ice was measured for the first time using a spectroscopic technique newly developed. The mutual diffusion process of proton (H+) and deuteron (D+) in an H2O/D2O ice bilayer, which was pressurized to several tens of GPa (1 GPa~104 bar) and heated to 100-200 oC in a high-pressure optical cell, was monitored by time-dependent measurement of infrared reflection spectra. Applying high pressure produced a dense structure not allowing molecular diffusion, which is the dominant diffusion process in ice at atmospheric pressure. In "hot ice" realized at temperatures above 100 oC, the protonic diffusion was thermally activated and became the dominant diffusion process instead of the molecular one.
The diffusion coefficient at 127 oC and 10 GPa was determined to be 10-15m2/s larger by a factor of 105 than the value estimated for ambient pressure ice. Protons can move around in ice at a rate of 30 nm (1 nm=10-9 m) per second on average. In other words, the proton jumps successively into the neighboring water molecules every 10 ms (1 ms=0.001 s). The results were reported in Science (Vol. 295, No.5558, 15 February 2002).
Ice is a key material of protonics and can compare with silicon, which was a key material in electronics. Further study of the protonic diffusion in ice at extended high temperatures and pressures would construct the basis for protonics.