Development of novel cancer vaccines by applying materials science and immunology
A self-assembly method has been devised to incorporate cancer vaccines into "metal-organic structure (MOF)-gated mesoporous silica (MS)." The MS nanoparticles as the core can hold antigens and immunostimulants, and the coating of MOF is designed to protect the loaded components and achieve pH-controlled release. The developed cancer vaccine, when combined with programmed cell death-1 (PD-1) blockade therapy, creates a synergistic effect that promotes anti-tumor immunity and reduces the required dose of anti-PD-1 antibody by 90% in tumor-bearing mice. This therapy has been shown to induce an adaptive T-cell response and sustain tumor suppression.
Problems with immune checkpoint therapy and how to overcome them
Immune checkpoint inhibitors reactivate immune functions that had been suppressed by cancer cells, expanding the potential of cancer immunotherapy. However, they have response rates of only 10-40%, are extremely expensive, and have problematic immune-related side effects. To overcome these problems, a method has been proposed to combine with cancer vaccines to enhance the immune response. In this study, we focus on MS@MOF-based cancer vaccines, which can effectively protect and control the release of loaded antigens and soluble immunostimulants.
Hierarchical self-assembly, in vitro studies, in vivo studies using mice
The self-assembly conditions of MS@MOF and the immunostimulants loading conditions are optimized. We confirmed that MS@MOF can hierarchically encapsulate a variety of biomolecules, including ovalbumin (OVA, a model antigen), polyinosinic acid-polycytidyl acid (an immunostimulant), and anti-PD-1 antibody (a checkpoint inhibitory antibody), and that the degradation of MS@MOF depends on pH. In particular, the slow degradation under neutral conditions and the rapid degradation under acidic conditions (pH 5) are advantageous in preventing burst release of antigens and immunostimulants and facilitating their intracellular delivery. In mouse experiments, no obvious toxicity was observed, and the combination of MS@MOF cancer vaccine and PD-1 blockade therapy promoted antitumor activity, reducing the required dose of anti-PD-1 antibody by 90% compared to PD-1 blockade therapy alone.
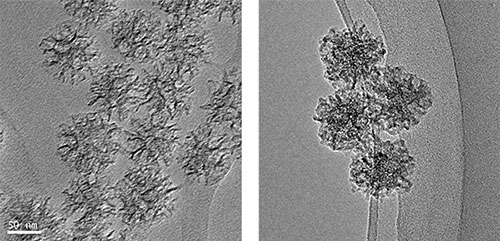 TEM images of MS (left) and MS@MOF (right)
TEM images of MS (left) and MS@MOF (right)
Broad application possibilities and bridging research
The developed inorganic/organic composite nanomaterial delivery systems are expected to be widely applicable to a variety of therapeutic agents ranging from peptides, nucleic acids, molecular immunostimulants, and chemotherapeutic drugs to imaging contrast agents. Since this study was conducted in mice, further studies are needed to advance this technology from pilot to clinical studies, and clinical trials are needed to demonstrate its safety and efficacy in humans.
We thank our collaborating researchers for their cooperation and advice. This work was supported in part by AIST and JSPS KAKENHI Grants JP22H03964, JP17K01399, JP26750162, and JP23700567. Part of the data is cited from the paper below.
Xia Li, Xiupeng Wang, Atsuo Ito, Noriko M. Tsuji. A nanoscale metal organic frameworks-based vaccine synergises with PD-1 blockade to potentiate anti-tumour immunity. Nature Communications. 2020; 11: 3858