―Glucose sensor without precious metals or interference filters, enabling miniaturization of blood gas analyzers―
Researchers) ITOH Tetsuji, Senior Researcher, HASEGAWA Yasuhisa, Deputy Director, Research Institute, Research Institute for Chemical Process Technology
- PB/G/PSS electrode replaces platinum electrode used in conventional working electrodes.
- Glucose can be measured without being affected by dissolved oxygen in blood or interfering substances such as vitamin C (ascorbic acid).
- Simplified glucose sensor structure contributes to downsizing and cost reduction of blood gas analyzers.
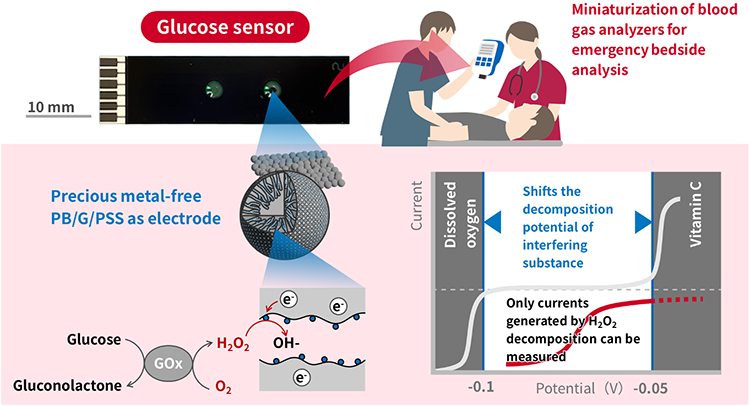
Overview of the developed glucose sensor
*Figure modified from Yoshida et al.(2025)
Researchers at AIST, Techno Medica Co. Ltd., Tohoku University, Fuji Silysia Chemical Ltd. and JEOL Ltd. have developed a compact sensor that can measure blood glucose levels (glucose concentration) without removing foreign substances from blood using an electrode without precious metals.
Blood glucose levels can be measured electrochemically by detecting hydrogen peroxide (H2O2), which is generated when glucose is oxidized by the enzyme (glucose oxidase: GOx). The H2O2 decomposed at a platinum (Pt) working electrode, and relating the current is used to determine the glucose concentration. However, since the decomposition potential suitable for detecting H2O2 overlaps with the decomposition potential of dissolved oxygen and vitamin C (ascorbic acid) in blood, it is necessary to remove these interfering substances beforehand for accurate measurement. In conventional sensors using Pt electrodes, H2O2 is decomposed at a decomposition potential unaffected by dissolved oxygen, and vitamin C is often removed by a interfering substance removal membrane. In this study, a working electrode(PB/G/PSS) was developed by immobilizing Prussian Blue (PB), which reacts with H2O2, into graphene-coated porous silica spheres (G/PSS). This electrode was used to shift the decomposition potential of dissolved oxygen and vitamin C, thereby enabling the development of a sensor capable of measuring H2O2 without interference from these substances. The developed glucose sensor can measure blood glucose concentration in a wider range of 0 to 270 mg/dL, including the concentration range of fasting blood glucose (glucose concentration: 70 to 100 mg/dL). Furthermore, we have confirmed that using PB/G/PSS for both the working and reference electrodes maintained sensor performance, leading to the successful development of a precious metal-free sensor.
The newly developed glucose sensor, which does not require a mechanism for removal of interfering substances, promotes miniaturization of blood gas analyzers that can measure blood glucose and lactate levels. Demand for such analyzers has been increasing in recent years. Moreover, since the sensors do not use precious metals such as Pt, the sensor contributes to stable supply and lower manufacturing costs.
Blood gas analyzers measure the partial pressure of oxygen and carbon dioxide in the blood, pH, and other parameters, and are indispensable in emergency medical care to determine the urgency of a patient's condition. In recent years, blood gas analyzers capable of simultaneously measuring blood glucose and lactate—useful for identifying the causes of consciousness disorders—along with blood gases have been introduced, enabling rapid measurement of a wide range of parameters. However, for emergency situations, there is a growing demand for compact and portable devices that enable more rapid testing. In addition, blood gas analysis uses arterial blood, which places a significant burden on patients. This is especially problematic in pediatric care, where devices capable of testing with small blood volume are needed.
The blood glucose level is measured based on the decomposition of H2O2, which is produced when glucose in the blood is oxidized by the enzymes GOx. The resulting electric current generated at the working electrode is then measured and correlated with the glucose concentration. In conventional glucose sensors, the decomposition potential of H2O2 overlaps with that of oxygen and vitamin C, which are interfering substances, and these interfering substances must be removed for accurate measurement. This removal mechanism complicates the sensor structure and prevents integration with other sensors, making it difficult to miniaturize the device. In addition, the use of the precious metal Pt for the electrode has also posed challenges in terms of manufacturing cost and material supply.
Journal: ACS Electrochemistry
Title of paper: Environmentally Sustainable, Noble-Metal-free Enzyme Sensors Capable of Functioning in the Presence of Ascorbic Acid
Authors: Akiko Yoshida, Zheng-Ze Pan, Mutsuhiro Ito, Kenichi Izawa, Yuka Minegishi, Yusuke Sakuda, Yukinori Noguchi, Yasuhisa Hasegawa, Tetsuji Itoh, and Hirotomo Nishihara
DOI: 10.1021/acselectrochem.5c00045