KAWANAMI Hajime, Chief Senior Researcher, Interdisciplinary Research Center for Catalytic Chemistry
- Developed technology to directly reproduce "formic acid" from carbon dioxide, a byproduct of hydrogen production
- Discovered a solvent (HFIP) that inhibits the decomposition rate of formic acid while enhancing formic acid formation.
- Formic acid enables carbon-neutral hydrogen storage and production system
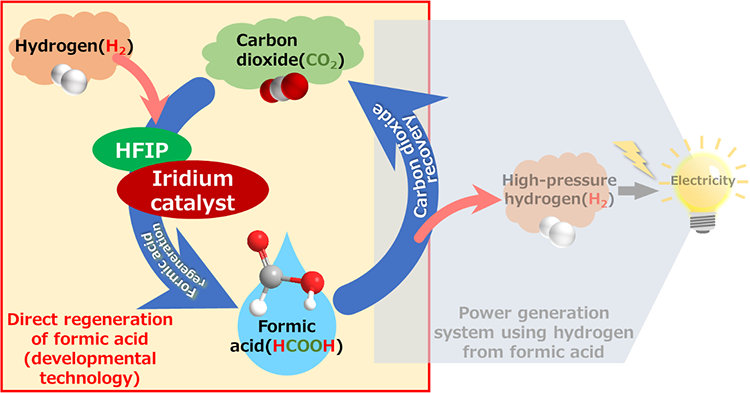
Overview of hydrogen storage and production system using formic acid
With the goal of achieving a carbon-neutral society and eliminating greenhouse gas emissions by 2050, efforts are accelerating to utilize hydrogen as a key energy source. To achieve this goal, the technologies for hydrogen storage, transportation, and reproduction are actively being developed in Japan, including high-pressure hydrogen, liquid hydrogen, and liquid organic hydrogen carriers (LOHCs) such as methylcyclohexane. One example is the transportation of hydrogen produced from lignite coal overseas to Japan, where it is used as liquid hydrogen in fuel cell vehicles, trains, forklifts, etc. Demonstration tests for these applications are already underway.
On the other hand, the use of high-pressure and liquid hydrogen, for example, presents challenges. It requires specialized equipment and heavy containers for safe storage and transportation, which pose safety risks and increased costs, limiting widespread adoption.
A researcher at AIST, in collaboration with the University of Tsukuba, has developed a highly efficient method for the direct formic acid synthesis from carbon dioxide and hydrogen.
Formic acid has attracted significant attention as one of the promising hydrogen carriers. In the conventional method, formic acid is first produced from carbon dioxide and hydrogen as a stable “formate salt" under basic conditions and later, the “formate salt” is converted to formic acid through acid treatment. However, these methods involve multiple steps to manage the generated heat and to remove by-products, leading to high production costs, which complicates the cost-effective supply of hydrogen.
In this study, we have developed a simple and efficient method for the direct synthesis of formic acid from carbon dioxide and hydrogen using the iridium catalyst in hexafluoroisopropanol (HFIP). Until now, direct synthesis with iridium catalysts faced challenges due to the rapid decomposition of formic acid into hydrogen and carbon dioxide in water. In contrast, we discovered that HFIP inhibits the formic acid decomposition and increases the formation rate of iridium hydride complexes, the key intermediates in the synthesis, by more than four times compared to that of water.
This breakthrough enables the direct, efficient production of formic acid without the need for formate intermediates. Furthermore, this achievement paves the way for formic acid to be used as a sustainable hydrogen source. By integrating it with AIST’s flow-based power generation system (please refer to the previous press release), this innovation could accelerate the development of carbon-neutral hydrogen storage and production solutions.
Journal: Organometallics
Title of paper: Direct formic acid production by CO2 hydrogenation with Ir complexes in HFIP under supercritical conditions
Authors: Seo Ono, Ryoichi Kanega, Hajime Kawanami
DOI: doi.org/10.1021/acs.organomet.4c00229