Department of Materials and Chemistry
Developing a Method for Converting PET
(Polyethylene Terephthalate) Bottles to Raw Materials
at Ambient Temperature
- TANAKA Shinji, NAKAJIMA Yumiko
Interdisciplinary Research Center for Catalytic Chemistry
Released: November 8, 2021
Efficient conversion of PET resin into raw materials at ambient temperature
We have developed a catalytic reaction that efficiently converts PET resin into raw materials using a new depolymerization approach. The reaction temperature was successfully reduced from about 200°C with the conventional method to ambient temperature. The developed approach potentially reduces costs of "Bottle-to-bottle" recycling of PET bottles.
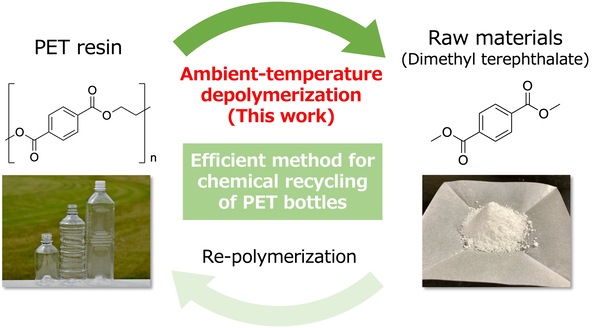 Overview of chemical recycling of PET bottles using our approach
Overview of chemical recycling of PET bottles using our approach
High reaction temperatures in chemical recycling pose a major challenge
Recently, environmental pollutions caused by plastic waste have become a serious issue in a modern society. Polyethylene terephthalate (PET) resin is an indispensable plastic material used as bottles for food/drinks and as fibers, and there is an urgent need to develop fundamental technology for recycling it efficiently. Chemical recycling, which chemically decomposes PET resins into small molecules, enables the reproduction with the same quality as the original PET products. However, this method is costly because a high temperature of about 200°C is required for the depolymerization process. Reducing the reaction temperature by a large extent is highly desired.
Full depolymerization of PET at ambient temperature promoted by trapping by-products
The key idea was to promote the reaction by capturing ethylene glycol, a by-product of depolymerization , with dimethyl carbonate and converting it to ethylene carbonate. As a result of experiments, about 90% of PET bottle flakes were depolymerized at ambient temperature for three hours, and the flakes were fully depolymerized at 50°C. The dimethyl terephthalate produced by this reaction is easily isolated with high purity (> 99%) and is reusable as raw materials for PET. The by-product, ethylene carbonate, can also be used as an electrolyte solution in lithium-ion batteries. Furthermore, the catalyst, lithium salt, precipitates as an insoluble solid after the completion of the reaction, and thus can be easily separated and recovered.
Improving the catalyst and scaling up the reaction to establish PET chemical recycling technology
Aiming toward the industrial application of this recycling method, we will extend the research for improving the catalyst, scaling up the reaction, and its applicability with various PET-containing products. We will also develop catalysts for recycling various types of plastic materials other than those with PET resins.
Contact for inquiries related to this theme
Silicon Chemistry Team, Interdisciplinary Research Center for Catalytic Chemistry
TANAKA Shinji, Senior Researcher
NAKAJIMA Yumiko, Leader, Team
AIST Tsukuba Central 5, 1-1-1 Higashi, Tsukuba, Ibaraki 305-8565 Japan
E-mail: irccc_silicon1-ml*aist.go.jp (Please convert "*" to "@".)