The researchers have developed a technique to evaluate the toxicity of chemicals in an easy and rapid manner, utilizing the fact that the RNA degradation rate in cells decreases when toxicity is present. In this technique, fluorescence probes are introduced into human cells and the rate of RNA degradation in the cells is determined by measuring the change in fluorescence intensity.
 |
|
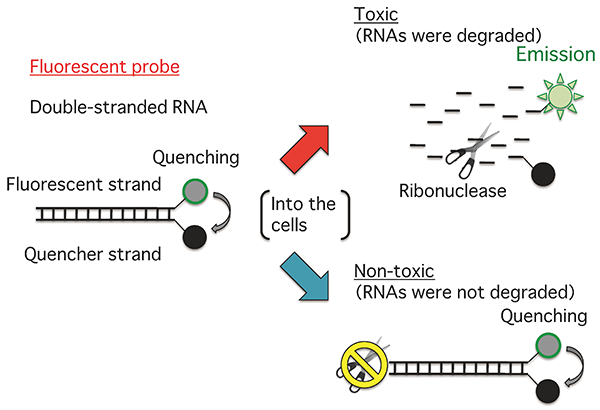
The fluorescence intensity of the probe increases in response to RNA degradation. |
The researchers have conducted a detailed molecular biological study of the change in the state of human cells when the cells are exposed to chemicals, and discovered that the rate of RNA degradation is decreased by the presence of toxic chemicals. They designed and synthesized a fluorescent probe, with a fluorescent dye on one of double-stranded RNA chains and a quencher dye on the other chain. In a system with a toxic chemical, the rate of RNA degradation is low and fluorescence remains quenched. However, fluorescence is emitted earlier in a system without such chemical addition, and there is a clear difference in the intensity of fluorescence between the two systems, making it possible to determine the toxicity of the added chemical in two hours, 1/4 of the time required for cell testing.
For the safe use of chemicals, it is necessary to evaluate their effect on the human body. The biological effects of chemicals have been evaluated by animal testing; however, the sale of products developed based on animal testing has been totally prohibited by European regulations. Thus, cell testing procedures are being developed as alternatives to animal testing. Current studies of cell testing focus on cell death, but such testing requires more than a half day to detect toxicity, and no appropriate cell testing procedure was established. For these reasons, there has been no significant reduction in animal testing.
The researchers will develop applications of the novel technique in a wide range of areas such as drugs and functional chemicals used for renewable energy, in addition to the toxicity evaluation of chemicals in the environment.