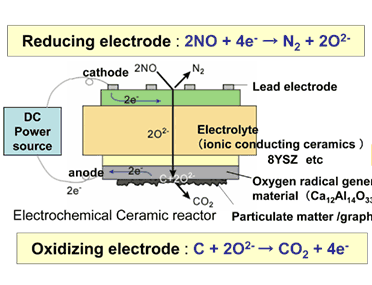
The Advanced Manufacturing Research Institute (AMRI), the National Institute of Advanced Industrial Science and Technology (AIST), an independent administrative institution, has developed leading the world an innovative electrochemical ceramic reactor which makes it possible to simultaneously decompose NOx and PM which give serious hurdle to diesel engine of high efficiency toward the practical use of a breakthrough ceramic reactor for exhaust gas cleanup characterized by low energy consumption and zero-emission (Fig. 1).
The technology allows reduction of NOx under coexisting oxygen and oxidation of PM in continued and simultaneous mode, in contrast to the conventional filter system, promising the development of high efficiency module for diesel emission cleanup.
-
The diesel engine has been known to be an internal combustion engine of high energy conversion efficiency, but its exhaust gas includes NOx to cause global warming and acidic rain as well as PM causing respiratory problems. The AMRI-AIST has already succeeded in selectively cleaning NOx in high temperature exhaust gas in the presence of oxygen through the electrochemical reactions in a ceramic reactor, and also reported operation of electrochemical reactor based on waste heat power generation, for the effective utilization of energy. The present technology concerns direct decomposition of solid carbon with a ceramic reactor to clean up diesel engine exhaust gas.
-
The AMRI-AIST has prepared cells based on ion conducting ceramics, as shown in Fig. 1, taking into consideration of reductive reaction for NOx in the electrochemical ceramic reactor and oxidative reaction at the electrode so as to directly oxidize solid carbon. After trying with various materials, effective direct decomposition of solid carbon has been successfully achieved by using calcium aluminate (Ca12Al14O33) for oxidizing electrode to promote the generation of oxygen radical through electrical field control.
-
The present technology decomposes NOx and PM in the same electrochemical reactor, suggesting the feasibility of a new diesel exhaust gas cleanup system to replace the DPF filter. Moreover, the technology may be applied to chemical industry process with oxidation and reduction controlled.
 |
 |
|
Fig. 1. Principle diagram of operation
|
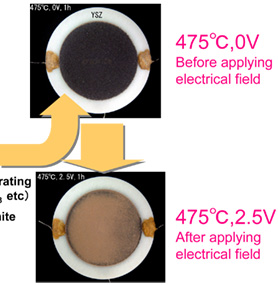
Fig. 2. Electrochemical PM decomposition with a ceramic reactor
|
For supporting a sustainable society meeting both efficient energy utilization and environment conservation, it is necessary to use high efficiency system by upgrading the materials function. The diesel engine has been known to have enhanced energy conversion efficiency from fossil fuel to power, but it has serious demerit: continuous cleanup of NOx with catalyst is hardly available because of high oxygen concentration in the exhaust gas. Moreover, it is urgently requested to remove PM (particulate matter based on carbon) simultaneously.
In the conventional technology, hazardous substances such as NOx is reductively decomposed by using active catalyst, such as metal three-way catalyst, but solid substance such as PM is difficult to decompose chemically, and has to be decomposed physically by adding a filter system. Filters are to be replaced and rinsed regularly. It is needed, therefore, to develop a technology for oxidatively decomposing hardly combustible solid carbon and hydrocarbon of greater molecular weight fully and continuously.
For simultaneously carrying out oxidative decomposition and reductive decomposition, electrochemical reaction control is available. In particular, for cleaning high temperature exhaust gas, it has been successfully achieved to promote reductive decomposition in the presence of oxygen and with lower power consumption by improving molecular selectivity at the electrode. In this case, as the reductive reaction is promoted by taking in and giving out oxygen molecules from NOx to solid electrolyte in the electrochemical cell (oxygen pumping), active oxygen is released in the reaction process. It may be expected that with the cell construction effectively utilizing these reactions, PM and solid carbon are oxidatively burnt, and noxious substances in exhaust gas are decomposed simultaneously and continuously.
Recently, it has been described that certain calcium aluminate materials can release active oxygen radical under electrical field. These materials are expected to oxidize solid carbon with active oxygen radical, though the decomposition of PM by use of these materials has not yet been reported. The present study has demonstrated that solid carbon can be decomposed continuously by combining an electrochemical ceramic reactor based on oxygen ion conduction by electrical field with oxidizing agent in electrical reaction field.
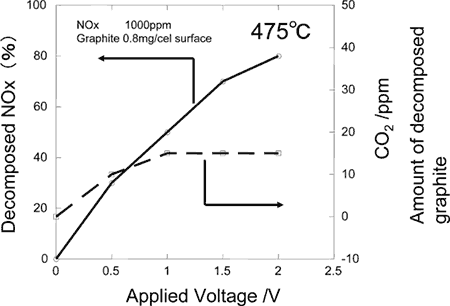
The electrochemical ceramic reactor is constructed by building on zirconium oxide ceramic substrate (8YSZ), oxygen ion conductor, a catalytic ceramics electrode composed of calcium aluminate for catalysis of combusting solid carbon and nickel oxide, catalyst for reducing NOx mixed with zirconium oxide (Fig. 1). With this reactor, electrical field is applied to the electrode under high temperature atmosphere to confirm burning of solid carbon through the electrochemical reactions. It has been found, then, on applying electrical field at 475 °C, carbon coated on the surface is decomposed to be removed directly (Fig. 2). Furthermore, when NOx is brought to coexistence, it has been possible to decompose electrochemically both solid carbon and NOx simultaneously (Fig. 3). Besides, dispersing calcium aluminate, such as Ca
12Al
14O
33 over the oxidizing electrode, accelerates the rate of electrochemical oxidizing reaction at the electrode (Table 1).
 |
|
Fig. 3. Simultaneous decomposition of solid carbon (PM) and nitrogen oxide (NOx) in a ceramic reactor: carbon and nitrogen decomposition vs. applied voltage. |
Table 1. Oxidizing electrode (anode) materials vs carbon decomposed at 475°C.
|
Electrode Materials
|
Carbon Decomposed (mol/cm2-h)
|
|
Pt + 8YSZ (zirconia)
|
0.3 x 10-5
|
|
Ag + 8YSZ (zirconia)
|
0.7 x 10-5
|
|
Ca12Al14O33 + 8YSZ (zirconia)
|
1.3 x 10-5
|