Kenji Koga (Senior Researcher) of the Physical Nano-Process Group, the Nanosystem Research Institute (Director: Tomohiko Yamaguchi), the National Institute of Advanced Industrial Science and Technology (AIST; President: Ryoji Chubachi) and Makoto Hirasawa (Senior Researcher) of the Advanced Manufacturing Research Institute (Director: Masanobu Awano), AIST have developed a technology for forming noble metal–oxide heterojunction nanoparticles.
Oxidation of an alloy nanoparticle comprising a noble metal and a base metal results in separation to the noble metal and the base metal oxide because only the base metal is oxidizable. The researchers demonstrated using a model of noble metals and nickel oxide (NiO) that the unidirectional growth of the oxide nucleated on alloy nanoparticles leads to generations of noble metal–oxide heterojunction nanoparticles. The novel technique allowed us to make conjunctions between a noble metal and an oxide at the nanoscale, without using complicated chemical processes. New functions enabled by connecting different types of nanoparticles are anticipated.
Details of the results have been published online on November 11, 2014 in a scientific journal, Materials Research Express, published by IOP Publishing in the U.K.
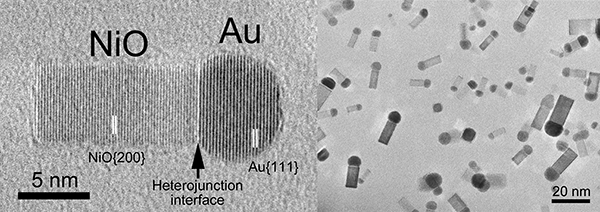 |
TEM images of the matchstick-shaped nanoparticles made of conjunction between gold (Au) and nickel oxide (NiO)
A Au nanoparticle and a NiO nanorod are connected so as to align the Au {111} lattice plane in parallel with the NiO {200} lattice plane. |
In recent years, a number of researchers around the world have been attempting to make junctions between nanoparticles of different materials. The aim of this research direction has been not only to integrate the individual functions into a single nanoparticle, but also to promote the emergence of new properties, such as catalytic activities, originating from the interfacial parts, and to achieve synergistic effects caused by electronic interactions through the interface. However, it is not so easy to prepare junctions between different types of nanoparticles. Solution-base synthesis techniques involving complicated chemical reactions have often been used to achieve nanoparticle conjugation, but there are problems including use of dangerous raw materials such as metal carbonyl compounds. In addition, clean surfaces are required when nanoparticles are applied to electronic devices, gas sensors, etc. Therefore, methods for clean production of heterojunction nanoparticles, preventing surface contamination, have been desired.
AIST has developed techniques for producing nanoparticles in the gas phase and structural characterizations using transmission electron microscopy (TEM) through works elucidating size- and temperature-dependent structural transitions in gold (Au) and silicon (Si) nanoparticles. With the help of these techniques, the researchers aimed to elucidate the oxidation processes of alloy nanoparticles composed of a base metal and a noble metal in the gas phase. Generation of noble metal–oxide heterojunction nanoparticles is expected by oxidizing the base metal components in the alloy nanoparticles, because the noble metal, such as Au, and platinum (Pt) in the alloy is less oxidized. However, systematic research has not been performed to date to reveal the relationship between the oxidation conditions and the resultant nanoscale morphologies.
This work was supported in part by a Grant-in-Aid for Scientific Research (C) (FY2010 to 2012) and a Grant-in-Aid Fund (C) (FY2014 to 2016) of the Japan Society for the Promotion of Science.
The alloy nanoparticles targeted in the present research were comprised of nickel (Ni), a typical base metal, and a noble metal, Au or Pt. The researchers made Ni–Au alloy or Ni–Pt alloy nanoparticles generated in helium (He) gas by laser ablation and oxidized in the gas phase. Morphologies of the oxidized particles were investigated using TEM. The alloy nanoparticles were heated in He gas flow through a quartz tube heated by a tube furnace, and also thermally oxidized by oxygen (O2) gas that was mixed into the flow. Here, the researchers chose two different oxidation methods as schematically shown in Fig. 1. Figure 1(a) shows the oxidation process wherein the O2 gas is mixed into the He gas flow carrying the alloy nanoparticles prior to heating them to the desired temperature. Figure 1(b) displays the process wherein the O2 gas and the particles drifting in the He gas are heated separately to the desired temperature and then mixed together quickly. More violent oxidation is expected in the latter process. The processes in Figs. 1(a) and 1(b) are called mild and rapid oxidation, respectively.
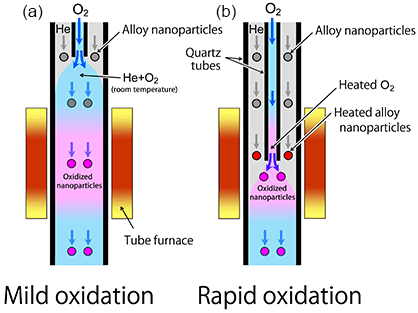 |
|
Figure 1 : Two different conditions for the nanoparticle oxidation |
Figures 2(a) and (b) are TEM images of the particles formed by mild oxidation (a) and rapid oxidation (b) of Ni–Au alloy nanoparticles (Au atomic concentration of 20 at.%) when the tube furnace is set at 600 °C. The mild oxidation process resulted in the formation of core–shell type nanoparticles, in which Au is encapsulated by NiO. This shape was formed through the previously reported process in which the alloy nanoparticle surfaces were oxidized uniformly. In contrast, the rapid oxidation gave rise to particles with matchstick-like morphology in which a Au nanoparticle is joined onto the tip of a NiO square-rod-shaped nanoparticle (nanorod). This form of the asymmetric combination of a Au nanoparticle and a NiO nanorod (Au–NiO nanorod) is promising for potential applications to gas sensors, catalysts, etc., because their interfacial plane is exposed to the nanoparticle surface.
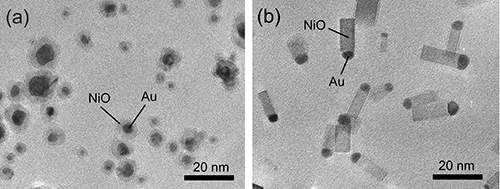 |
Figure 2 : TEM images of (a) Au–NiO core–shell particles formed by mild oxidation and
(b) Au–NiO nanorods formed by rapid oxidation of Ni–Au alloy nanoparticles (Au atomic concentration: 20 at.%)
The oxidation temperature is 600 °C, the partial pressure of oxygen is 533 Pa, oxidation time is approximately 0.01 s. |
Experiments under controlled O2 partial pressures revealed a completely new oxidation mechanism underlying the generation of the Au–NiO nanorods (Fig. 3). At the initial stage of alloy nanoparticle oxidation, a small island of NiO is formed on the alloy particle surface. Under the rapid oxidation conditions, the island grows unidirectionally, resulting in the buildup of a NiO nanorod on the alloy particle. During the nanorod growth, the Ni atoms are extracted from the alloy particles condensing Au in them until only Au is left at the tip of the nanorod. Even under the rapid oxidation conditions, at temperatures lower than 400 °C the core–shell nanoparticles were formed instead of the Au–NiO nanorods.
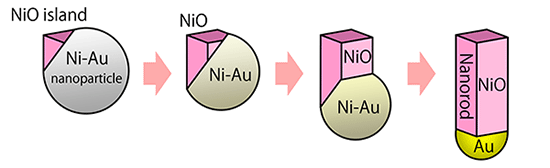 |
|
Figure 3 : Formation process of Au–NiO nanorods by rapid oxidation of Ni–Au alloy nanoparticles |
Figures 4(a) to (d) are TEM images of Au–NiO nanorods obtained by rapid oxidation of Ni–Au alloy nanoparticles of various compositions. Figures 4(e) and (f) are TEM images of Pt–NiO nanorods produced by rapid oxidation of Ni–Pt alloy nanoparticles with two different compositions. By changing the types and compositions of the noble metal in the alloys, the size ratio of the noble metal versus NiO is easily controllable.
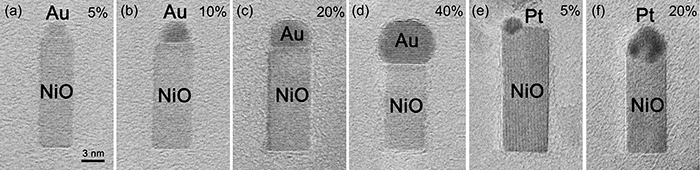 |
Figure 4 : TEM images of Au–NiO nanorods (a–d) and Pt–NiO nanorods (e, f) with different relative sizes of the noble metal and NiO
The value denotes the atomic concentration (at.%) of the noble metal in the raw alloy. The scale is common in all images. |
As described above, clean and continuous generation of nanoscale noble metal–oxide particles could be achieved by unidirectional oxide growth induced under the rapid oxidation conditions. Heterojunction nanoparticles made of combinations of noble metals and oxides of copper, tin, aluminum, and cobalt were also generated, and their shapes were found to be dependent on the formation conditions, the crystal structures of oxides, etc.
The next stage of this research is to understand more deeply the nanoscale oxidation mechanisms through generation of more different types of noble metal–oxide heterojunction nanoparticles. In addition, the researchers plan to evaluate gas sensing and catalytic properties, etc., of noble metal–semiconductor oxide heterojunction nanoparticles.