- Efficient catalyst for oxidation reaction using metal-complex-type organic nanotubes -
Masaru Aoyagi (Researcher) and Masaki Kogiso (Senior Researcher), Organic Nanotube Team (Leader: Masumi Asakawa), the Nanotube Research Center (Director: Sumio Iijima) of the National Institute of Advanced Industrial Science and Technology (AIST; President: Tamotsu Nomakuchi), in collaboration with Hiroharu Yui (Associate Professor), Department of Chemistry, Faculty of Science Division1, the Tokyo University of Science (Chairperson, Board of Governors: Takeo Tsukamoto), and Tanmay Chattopadhyay (Assistant Professor), Panchakot Mahavidyalaya (Principal: Sanjib Chattopadhay), have discovered that nickel-complex-type organic nanotubes (Ni-ONTs) function as the catalyst for oxidation reactions of various organic compounds, indispensable for industries, in water at room temperature. Ni-ONTs were synthesized by the mass production method developed by AIST.
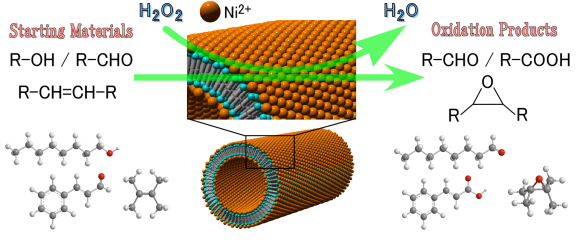
Ni-ONT can be synthesized by the simple operation of mixing inexpensive amphiphilic molecules, glycylglycine connected with a fatty acid, and nickel salt in a solvent. Because all nickel ions are exposed on the inside and the outside surfaces of the nanotube, Ni-ONT is expected to provide excellent catalytic sites. (Fig. 1) Since Ni-ONT is solid in water, it can be easily recovered through filtration after the catalytic reaction and is also recyclable. Therefore, Ni-ONT is expected to contribute to green innovation.
Details of the results will be published online in Green Chemistry, a scientific journal of the Royal Society of Chemistry on April 1, 2011.
 |
|
Figure 1: Oxidation reaction proceeds efficiently on the nickel ions exposed on the surface of nanotube. |
In order to realize organic reaction processes (green processes) giving a less environmental impact, the catalysts that enable reactions to proceed efficiently under mild conditions are indispensable. Especially solid-supported catalysts that can be easily separated from products after reaction are highly useful. However, the high cost has been a problem due to the fact that many of the solid-supported catalysts need noble metals and heat-treatment for their formation. In addition, many heterogeneous reactions using a solid-supported catalyst are carried out in an organic solvent. Considering waste solvent treatment, the reaction processes that can be implemented in water, not in an organic solvent, are desired from the viewpoint of the environmental impact.
Over the past ten years, AIST has been conducting research on syntheses of organic nanomaterials such as nanofibers and nanotubes by self-organization of amphiphilic molecules in a solution. The amphiphilic molecules are synthesized from reproducible natural origin materials. In 2008, we pioneered in developing the method for mass production of metal-complex-type organic nanotubes and are presently developing applications of the organic nanotubes.
A part of this study was supported by Grants-in-Aid for Scientific Research (Young Scientists B) (FY2010-FY2011) of Japan Society for the Promotion of Science.
Ni-ONT can be obtained within 3 hours by adding an aqueous solution of a nickel salt to an alcohol suspension of the amphiphilic molecules synthesized by combining glycylglycine and a fatty acid. (AIST press release on October 24, 2008) Both glycylglycine and the fatty acid are low environment impact materials produced easily from reproducible resources. Nickel is the fifth most abundant element on the earth and inexpensive compared with the noble metals used for many catalysts. Therefore, the cost of Ni-ONT production is thought to be limited. In addition, since nickel ions have been known to indicate catalytic activity in organic oxidation reactions, Ni-ONT (Fig. 2 below) that has a nanotube structure composed of single bilayer membrane with nickel ions fixed on the surface is expected to function as a heterogeneous catalyst with a large specific surface area.
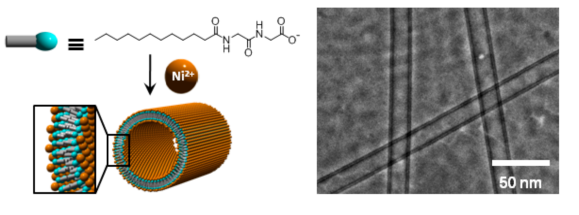 |
Figure 2 : Schematic illustration and electric microscope image of Ni-ONT
Nickel ions are attached to the surface of single bilayer membrane nanotube. |
Investigating the use of Ni-ONT as a catalyst for organic oxidation reactions from this perspective, we have found that the oxidation reaction proceeds by the simple operation of dispersing Ni-ONTs into an aqueous solution of hydrogen peroxide, and stirring it at room temperature after adding an organic compound. (Fig. 3) As shown in Fig. 4, Ni-ONT is applicable to a wide variety of oxidation reactions of organic compounds. In addition, hydrogen peroxide is an environment-friendly oxidizing reagent.
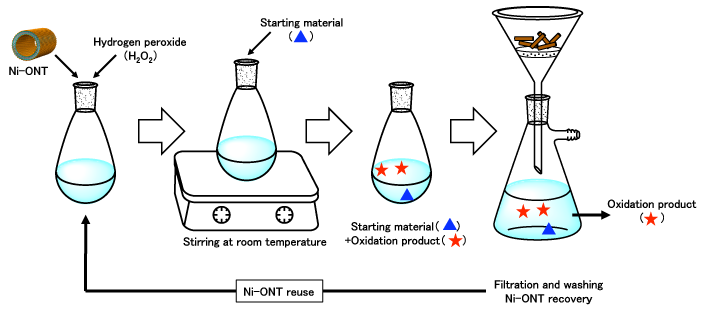 |
Figure 3 : Flow chart of oxidization reaction
First, Ni-ONTs are dispersed in an aqueous solution of hydrogen peroxide. An organic compound is added to the solution and
the solution is stirred at room temperature. |
 |
|
Figure 4 : Examples of starting materials and reaction products of oxidization reactions catalyzed by Ni-ONT |
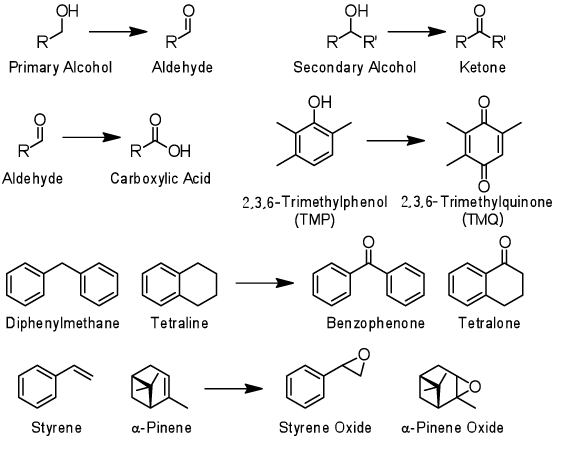
Many of the oxidation reactions in conventional industrial processes have problems such as 1. necessity of heating operation, 2. necessity of organic solvent addition, 3. use of organic peroxyacids having explosiveness as an oxidizing reagent, toxic heavy metals, and strong acids, 4. formation of dangerous peroxides during the reaction, 5. production of halogen-containing waste due to the use of halogen compound as a starting material. For example, the ordinary epoxidation processes of olefin compounds including α-pinene and styrene have more than one problem mentioned above. On the other hand, the oxidation reactions using the developed Ni-ONT catalyst do not have any of these problems. The oxidation reaction proceeds by dispersing Ni-ONT catalyst into an aqueous solution of hydrogen peroxide, i.e. oxidizing reagent, adding a starting material, and stirring the solution at room temperature. Therefore, there are advantages as follows. 1. The energy cost can be reduced because no heating operation is needed. 2. Waste solvent can be treated easily, as no organic solvent is used, 3. Hydrogen peroxide is used as an oxidizing reagent, and organic peroxyacid, heavy metals, and strong acids are not used. 4. It is safe as no dangerous peroxide is produced. 5. The waste treatment cost can be reduced, because no halogen-containing waste was generated.
It is easy to refine the reaction product, as Ni-ONT catalyst and reaction solution can be separated by filtration after the reaction, and at the same time Ni-ONT catalyst can be recovered. In addition, reactivation of the catalyst by washing is possible, and the catalytic activity does not decline by being recycled at least five times. (Fig. 5)
Among the compounds produced by the oxidation reaction using Ni-ONT catalyst, TMQ as an intermediate of vitamin E synthesis, benzophenone as an ultraviolet absorber (an initiator of photo-polymerization), tetralone as an agrochemical intermediate, and epoxy compounds in photo curable resins are widely used, and these synthesis reactions are very important processes for industries.
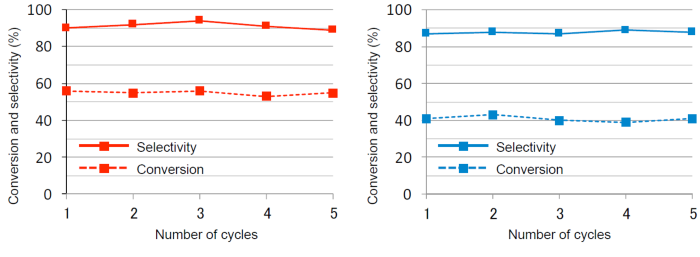 |
Figure 5 : Change in Ni-ONT catalyst activity for oxidation reaction by recycling
Oxidation reactions of TMP (left) and styrene (right) (Reaction time : five hours) |
We will conduct research on the improvement in efficiency and durability of metal-complex-type organic nanotube catalysts, and progress the demonstration to realize environment-friendly oxidation reaction processes through cooperative research including sample supply.