– Detector uses a "butterfly-shaped" organic nitrogen compound that is sensitive to double-bonded gas components –
Sites that use fluorine-containing etching gases such as C4F6 and C5F8 are equipped with gas leak detectors to manage gas concentrations in rooms when the gases are used. However, fluorine-containing gases included in refrigerants and cleaning agents sometimes trigger false alarms of the detectors. The newly developed detection agent reacts with the double bonds of the etching gases, causing it to change color. Perfluorocarbons and similar gases that are used as refrigerants and cleaning agents, despite being fluorine-containing gases (including vapor of liquids), do not react with the detection agent, since they consist solely of single bonds, with no double bonds, and therefore do not generate false alarms, even during replacement of the refrigerant.
This selective detection principle is believed to apply to next-generation fluorine-containing etching gases with higher performance than C4F6 and C5F8 and other halogen-containing gases, and is expected to extend to a wide range of applications as a leak detection technology that detects only highly reactive gases whose adverse biological effects are a concern.
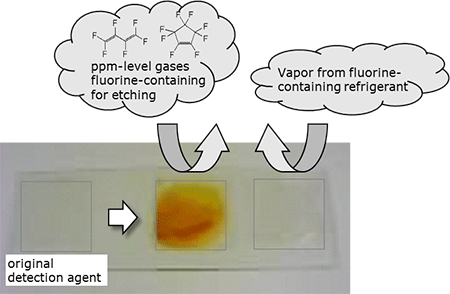 |
The developed detection agent (left) and changes in color when the agent comes into contact with fluorine-containing gases (right)
It can detect the fluorine-containing etching gases selectively and with high sensitivity. |
Advanced semiconductor fabrication processes use fluorine-containing gases such as C4F6 and C5F8 as etching gases in order to obtain a high selectivity ratio in semiconductor surface processing. Because of a concern for adverse environmental and biological effects of these etching gases, factories install a large number of gas detectors to detect any leakage of these gases quickly in an effort to keep the concentration of these gases in the environment at 2 ppm or less. However, the gas detectors in current use rely on high thermal decomposition and are also sensitive to vapors of fluorine-containing refrigerants and cleaning agents, leading to demand for a detection technology that can distinguish etching gases from the vapor of refrigerants and cleaning agents.
AIST has been developing techniques for detecting metal ions in liquids and specific gases in air by taking advantage of organic compounds and other agents whose characteristics change when they react with a trace amount of substances.
In this research, the researchers searched for an organic compound that changed color when selectively reacted with specific fluorine-containing gases, and worked on developing a detecting agent and a detector that used this organic compound.
The newly developed detection agent is a mixture of multiple substances, and the core of which are heterocyclic organic nitrogen compounds with two rings having a butterfly-like structure (for example, C7H12N2: 1,5-Diazabicyclo[4,3,0]non-5-ene), as illustrated in Fig. 1. The N=C-N part at the center of the molecule reacts with the double bond of the fluorine-containing gas molecules, creating a new conjugated molecule, and as a result, alter its absorption characteristics at UV and visible light wavelength regions.
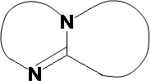 |
Figure 1: Molecular structure of the organic nitrogen compound which is the core of the detecting agent for etching gases
The molecular structure of the curved parts is -(CH2)n-, a hydrocarbon chain consisting of one or more carbon atoms. |
When the etching gas comes into contact with filter paper impregnated with the detection agent, the color of the filter paper changes from white to brown (its reflectance is reduced). This reaction is sensitive and fast enough to detect concentrations of only 2 ppm (0.0002%) of the etching gas (C5F8) and change color within one minute (about 4 % reflectance change in 1 minute) (Fig. 2). Moreover, because the degree of color change is proportional to the concentration of the etching gas from 0 to 50 ppm, the detector also indicates the concentration of the target gas.
On the other hand, perfluorocarbons and similar compounds used in refrigerants and cleaning agents are also fluorine-containing gases, but do not have double bonds. Therefore, when the detection agent comes into contact with refrigerant perfluorocarbon vapor, it does not react and does not change reflectance, even at concentrations exceeding 10,000 times the etching gas (Fig. 2). If the noise level (0.2 %) of reflectance is taken into account, the agent can be considered to have a selectivity factor of 200,000. The detection agent does not react with vapor of perfluoroethers either, which are used as refrigerants and cleaning agents.
The gas detectors that are currently in general use in semiconductor factories use pH indicators or similar means to detect hydrogen fluoride created by high thermal decomposition of the target gas. As a result, these detectors sometimes issue false alarms due to their inability to identify differences in the chemical structures of the etching gases and vapor of refrigerants (Table 1). Avoiding false alarms requires measures such as disabling the etching gas detectors whenever the refrigerant is replaced, thereby disrupting the etching process at the site. Because no such measures are needed when the developed detection agent is used, the agent is expected to contribute to increased productivity in semiconductor fabrication.
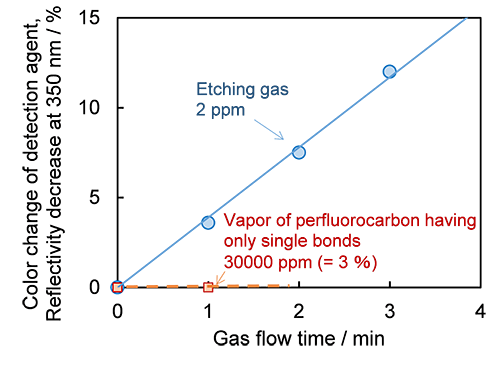 |
Figure 2: Changes in the coloration of the detection agent after introducing etching gas (C5F8) and perfluorocarbon refrigerant vapor
As an indicator of coloring, the vertical axis shows changes in reflectance of 350 nm, at which the color changes significantly. |
In order to demonstrate the usefulness of the detection using this agent, the researchers created a prototype etching gas leak detector (Fig. 3). Gas taken from the gas sampling tube (A) is fed into the reflectance measuring chamber (B), where it is brought into contact with the filter paper impregnated with the detection agent. By illuminating the filter paper with an LED (C) and measuring the intensity of the reflected light (that is, the change in color), trace amounts of etching gases can be detected (this technique is called "photoelectric photometry"). Because the prototype does not require a reactor for high thermal decomposition of the sample gas, it is possible to fit the prototype, including the results display panel (D), comfortably in a single briefcase, which is comparable in size to a conventional gas leak detector.
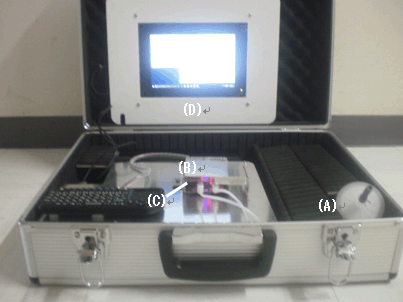 |
Figure 3: Prototype high-sensitivity etching gas leak detector
It consists of (A) gas sampling tube, (B) reflectance measuring chamber with
detection agent inside, (C) LED light source, and (D) results display panel. |
The detection agent takes advantage of the fact that when double-bonded carbon atoms bind to highly electronegative halogen atoms, the double bond is destabilized. Similarly, the new detection agent finds and reacts with the double bond of other halogen-containing gases (for example, 1,1,2,2-tetrachloroethylene and 1,2-dibromoethylene) as well as that of fluorine-containing gases. For the halogen-containing gases as well, those consisting of only single bonds, such as perfluorocarbon refrigerants, are chemically stable and non-toxic, whereas those having double bonds1 are often reactive and cause concern due to their adverse biological effects. It is therefore expected that use of this detection agent will also allow fast and selective detection of leakage of halogen-containing gases and liquids, or solutions containing them, whose adverse biological effects are of concern.
1 Including those in which a double bond is formed by reacting with the detection agent (for example, perfluorocarbons consisting of only single bonds in which some of the fluorine is replaced by hydrogen).
The researchers plan to pursue further improvement of the detection agent through demonstration in an actual factory environment. They also aim at practical use as a leak detector through optimization of the detection agent for different purposes and sophistication of the detection system, thereby contributing to the improvement of safety and production efficiency at manufacturing sites. The researchers will conduct "bridging" research aiming at practical applications in cooperation with companies with an interest in the developed technology, including companies that manufacture gas detectors and companies that manufacture halogen-containing gases and liquids (for example, next-generation fluorine-containing etching gas, raw material for plastics and functional materials, agricultural chemicals and pesticides, and pharmaceutical intermediates), as well as companies that use these products.
Studies in connection with the detection agent will soon be published in a European scientific journal, Tetrahedron Letters.