Haoshen Zhou (Prime Senior Researcher, Leader of Energy Interface Technology Group, and also Project Professor of social cooperation program “Advanced Battery Materials Technology” of the University of Tokyo), the Energy Research Institute for Energy Conversion (Director: Tetsuo Munakata) of the National Institute of Advanced Industrial Science and Technology (AIST; President: Ryoji Chubachi) , in collaboration with Fujun Li (AIST Postdoctoral Researcher and Project Researcher of the social cooperation program), Chichao Wu (Doctoral course student, University of Tsukuba), De Li (former AIST Postdoctoral Researcher), Tao Zhang (former AIST Postdoctoral Researcher), Atsuo Yamada (Professor and also Project Professor of the social cooperation program), School of Engineering, the University of Tokyo, and Ping He (Associate Professor), Nanjing University, has demonstrated a large reduction in overvoltage of air electrodes of lithium-air batteries by adding a small amount of water as catalyst to the organic electrolyte solution, DMSO.
Lithium-air batteries are expected to have a higher weight energy density than present lithium ion batteries theoretically because they use oxygen in the air for the electrochemical reaction. On the other hand, since lithium-air batteries have varieties of problems, they cannot be put into practical application right away. One of the main problems is that, since the electrochemical reaction between lithium and oxygen does not proceed ideally, the voltage difference between voltage gained by discharging and the voltage required for charging becomes high at about 1.0 V and the energy efficiency deteriorates.
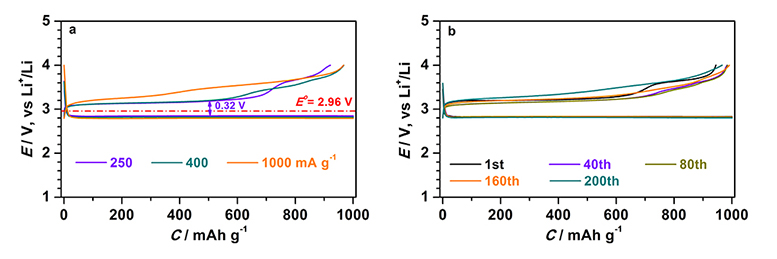
In this basic research focusing on the elucidation of reaction mechanisms of charging and discharging at air electrodes and the reduction of overvoltage, by using carbon, ruthenium, and manganese dioxide, and adding a small amount of water (about 100 ppm) to an organic electrolyte solution, DMSO, the researchers have confirmed that the overvoltage greatly decreases to about 0.21 V and the voltage difference between the discharging voltage and the voltage required for charging was only 0.32 V.
These results will be published online in a British international scientific journal, Nature Communications, at 18:00 (Japan Time) on July 24, 2015.
 |
|
Rate characteristics of the developed air electrode for lithium-air batteries (left) and its charge/discharge cycle characteristics (200 cycles) at current density of 500 mA/g (= 0.25 mA/cm2) (right) |
Recently, against the background of energy and environmental issues, electric cars have been popularized. At present, lithium ion batteries are installed in electric cars. However, the development of higher performance storage batteries enabling a much longer run is in high demand. The lithium-air battery has attracted attention as a post-lithium ion battery with a theoretical weight energy density of about 5-8 times as much as the present lithium ion batteries. However, there are issues such as the fact that little is known about the reaction mechanisms of charging and discharging at air electrodes, the overvoltage at charging becomes a high value larger than 1.0 V, and the charge/discharge cycle characteristics are inferior.
Zhou’s group in AIST has pointed out the expectation on large output power by using nano-structured electrode materials aiming at putting next generation lithium ion batteries in practical use (AIST press release on August 27, 2008) and has studied a new type lithium-air battery for electric cars with expectation of drastic improvement in weight energy density. (AIST press releases on February 24, 2009 and November 5, 2012). At present, Zhou’s group in AIST has been studying and developing the lithium-air battery, along with the study of lithium-sulfur batteries and sodium ion batteries, as next generation storage batteries.
Lithium-air batteries use oxygen (O2) in the air for the electrochemical reaction. In ideal reactions, the electron from the external circuit and the lithium ion (Li+) in the electrolyte solution reductively react with oxygen diffused to the air electrode and becomes lithium peroxide (Li2O2) in case of discharging, and in case of charging, Li2O2 is decomposed with an oxygen generating reaction and generates lithium ions and oxygen conversely. However, the overvoltage of Li2O2 oxygen generating reaction at the air electrode reaches a value higher than 1.0 V and carbon, catalyst and so on used for the air electrode become corroded. Therefore, use of carbon-free air electrodes for corrosion countermeasures and use of iodine ion etc. for countermeasures against overvoltage are being actively studied.
In this research, the researchers have focused on water, which is not utilized in non-aqueous lithium-air batteries. In the measurement system to evaluate the overvoltage of an air electrode, lithium iron phosphate (LiFePO2) is used as a negative electrode, DMSO added with a small amount of water (about 100 ppm) is used as an organic electrolyte solution, and carbon, ruthenium (Ru), and manganese dioxide (MnO2) are used as catalysts of an air electrode. In the battery of this composition, Li2O2 generated by discharging on the air electrode reacts with H2O, becoming solid state lithium hydroxide (LiOH) and hydrogen peroxide (H2O2) (Li2O2 + 2H2O = 2LiOH + H2O2). LiOH is decomposed into Li+, O2, and H2O by an oxygen generating reaction at low voltage and H2O2 is also decomposed into O2 and H2O by an oxidation-reduction reaction catalyzed by MnO2. In these reactions, H2O works as a catalyst by circulation without any consumption via intermediate LiOH. By using that air electrode, at the current density 250 mA/g based on the weight of carbon, Ru, and MnO2 of the air electrode, the overvoltage of charging and discharging are decreased to 0.21 V and 0.11V, respectively, and the voltage difference between the voltage gained by discharging and the required voltage for charging, becomes only 0.32 V. Furthermore, overvoltage of charging was reduced greatly at the current density of 500 mA/g and 1000 mA/g. In charge/discharge cycle testing at discharge capacity of 1000 mAh/g, stable charge/discharge cycle characteristics (200 cycles) could be obtained.
The researchers will investigate the optimization of composition and operational environment for the air electrode of lithium-air batteries and develop the electrolyte solution instead of DMSO. They will accumulate basic research of lithium-air batteries and focus on the development of a lithium-air battery with excellent performance.