Junji Akimoto (Leader) of Crystal and Materials Processes Group, Advanced Manufacturing Research Institute (Director: Nobumitsu Murayama) of the National Institute of Advanced Industrial Science and Technology (AIST; President: Tamotsu Nomakuchi), and Yoshito Gotoh (Leader) of Nano-Dynamics Analysis Research Group, Research Institute of Instrumentation Frontier (Director: Yoshio Akimune) of AIST, have developed a new high-capacity titanium oxide material (H2Ti12O25) for the negative electrodes of lithium ion secondary batteries in collaboration with Ishihara Sangyo Kaisha, Ltd. (ISK; President: Kenzo Oda).
While not employing lithium as a constituent element, the developed material realizes the same voltage (approximately 1.55 V vs. Li/Li+) as and a higher charge-discharge capacity per mass of oxide (225 mAh/g against 175 mAh/g) than lithium titanate (Li4Ti5O12) presently used in negative electrodes. In addition, because the hydrogen atoms in the material form a skeletal structure due to hydrogen bonding, the structure of the material is stable, and is not affected by the lithium insertion and extraction reactions during charging and discharging. The fact that lithium is not employed as a constituent element of the new material is also beneficial from the perspective of cost. Because of these, the new material is expected to contribute to the realization of increased capacity, longer life, and reduced costs in lithium ion secondary batteries used in electrically driven vehicles such as electric vehicles and hybrid vehicles.
 |
|
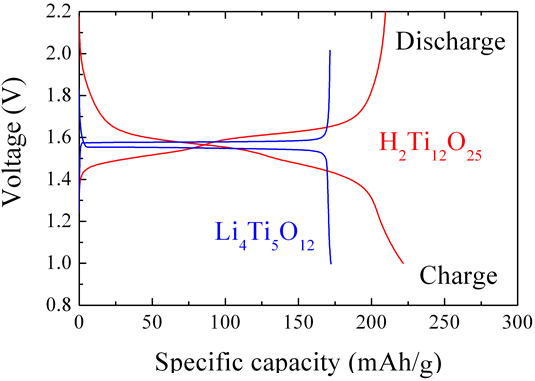
Figure 1 : Charge and discharge curves for developed titanium oxide negative electrode material (H2Ti12O25) and conventional lithium titanate negative electrode material (Li4Ti5O12) (Counter electrode: Metallic lithium; Current density: 50 mA/g) |
Because of their superior characteristics, such as high energy density, high voltage, and long cycle life, lithium ion secondary batteries are widely used in portable information devices including cellular telephones and notebook computers, and in devices for industrial applications. Full-scale use of large lithium ion secondary batteries in applications including automobiles, transport equipment, power storage, load leveling, industrial machinery, and machine tools is expected in the future. Given this, manufacturers, in particular Japanese battery manufacturers, are actively conducting research and development in this area and investing in production facilities.
For use in automotive applications, not only must the input and output characteristics of the batteries be improved and their energy density increased, it is also necessary to ensure safety and increase lifespan. Because of this, the use of oxides in the negative electrodes of the batteries is being studied. However, the use of lithium titanate, the current material, results in the issue of low energy density. The development of a high-capacity oxide which would ensure a high energy density at the same time as realizing the same high voltage as lithium titanate as a substitute material for use in negative electrodes was therefore highly desirable.
AIST has conducted research on the synthesis of titanium oxides using soft chemical synthesis, a low-temperature synthesis process, and evaluated the structures and properties of these compounds. During this research, a new titanium oxide compound (H2Ti12O25) was discovered, and studies were conducted of the method to synthesis it and its applicability as an electrode material for lithium ion secondary batteries.
ISK is a major manufacturer of titanium dioxide (titania) in Japan. It has developed and manufactures a variety of titania-related materials which are suitable for use in electronic materials, cosmetics, conductive materials, and photocatalysts, and for the main use in pigments. As part of these efforts, ISK has utilized its titania manufacturing technologies to develop lithium titanate as a new material, and has promoted the use of this material among users.
In a joint research for patent implementation conducted from FY2008 to FY2010, AIST and ISK have clarified the characteristics of the chemical bonding of the hydrogen atoms contained in the new titanium oxide discovered by the AIST and conducted chemical and electrochemical evaluations of its applicability as a material for use in the negative electrodes of secondary batteries, in addition to working to develop industrial manufacturing technologies.
At present, graphite carbon materials are the most widely used materials in the negative electrodes of lithium ion secondary batteries. Carbon materials display a low voltage (approximately 0.2 V vs. Li/Li+). If lithium cobalt oxide or a similar material is used for the positive electrode, the voltage of the battery can be increased (to approximately 3.7 V) and a high energy density can be achieved. However, batteries in which carbon materials are used in the negative electrodes display issues in terms of reliability including lifespan. For example, the capacity of the batteries declines if they are used for extended periods in high-temperature environments of 60°C or higher. By contrast, the use of lithium titanate (Li4Ti5O12), which is known to display reversible lithium insertion and de-insertion reactions, increases safety and enables the realization of a long lifespan. Therefore the compound was investigated as a next-generation, high electrical potential negative electrode material. However, this titanium oxide possesses a high voltage at approximately 1.55 V vs. Li/Li+, preventing the achievement of the high voltage characteristic of lithium ion secondary batteries. In addition, lithium titanate contains lithium as a constituent element, which does not contribute to the lithium insertion and de-insertion reactions, and there was concern that a significant increase in the volume of use of lithium in future with the widespread use of large lithium ion secondary batteries would result in increased costs.
The new titanium oxide discovered by AIST, H2Ti12O25, displays the same voltage as lithium titanate, and is expected to realize a high level of safety as a negative electrode material. Because its capacity is higher, a high energy density can be achieved in batteries using it. In addition, because it does not contain lithium as a constituent element like lithium titanate, its use is expected to reduce the cost of batteries.
The new titanium oxide is synthesized by a soft chemical synthesis, a method that retains the skeletal structure of the starting material, but modifies the chemical composition of the compound. Sodium titanate, Na2Ti3O7, is used as the starting material. First, the compound is subjected to acid treatment at a temperature of 60°C to produce the precursor, H2Ti3O7. Following this, the material is heated to temperatures of 200-300°C to enable the production of the target compound, the new titanium oxide H2Ti12O25.
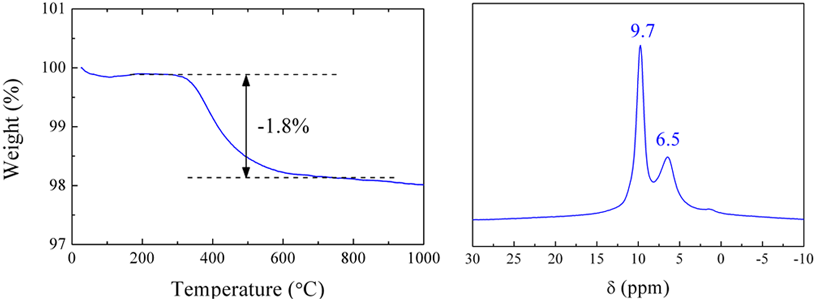
Figure 2 shows the result of a thermogravimetric analysis of the new titanium oxide and its 1H solid state NMR spectrum. The amount of dehydration estimated from the decrease in weight is consistent with the chemical composition H2Ti12O25. In addition, the solid state NMR spectrum shows that the hydrogen atoms contained in the compound are firmly fixed in a crystal structure by hydrogen bonding, and will not detach easily. As two peaks are observed, two types of occupied site exist in the structure. In addition, the density of the compound measured using a gas pycnometer was 3.50 g/cm3. Though the compound contains hydrogen atoms, this value is almost identical to that for the conventional lithium titanate (3.49 g/cm3).
 |
|
Figure 2 : (a) Thermogravimetric analysis and (b) 1H solid state NMR spectrum of new titanium oxide |
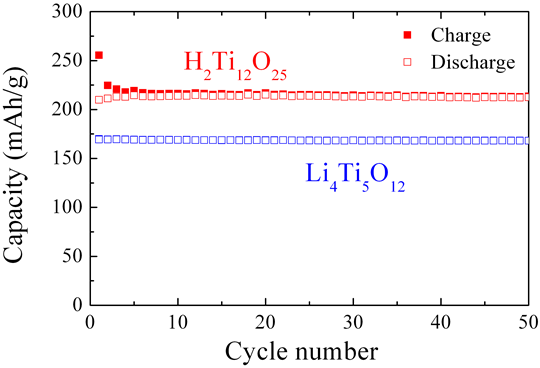
Figure 3 shows the charge-discharge cycle characteristics of the new titanium oxide and the conventional lithium titanate (LT-017, manufactured by ISK) measured under the same conditions. Though the new titanium oxide displayed an initial irreversible capacity, with measurements of 255 mAh/g for charge capacity and 210 mAh/g for discharge capacity at room temperature during the first cycle, in the tenth cycle it displayed basically reversible charge and discharge capacity, with figures of 216 mAh/g for charge capacity and 214 mAh/g for discharge capacity. After 50 cycles, charge capacity was 213 mAh/g and discharge capacity was 212 mAh/g. The new material displayed an excellent cycle characteristic equivalent to that of the conventional lithium titanate, while it maintained a high capacity of over 200 mAh/g. This indicates that the existence of hydrogen as a constituent element of the new titanium oxide does not present any issues in terms of its use as an electrode material. In addition, capacity will be increased by approximately 30% against the conventional material.
 |
|
Figure 3 : Room temperature charge-discharge cycle characteristics of new titanium oxide and conventional lithium titanate (LT-017, manufactured by ISK) (Counter electrode: Metallic lithium; Current density: 50 mA/g) |
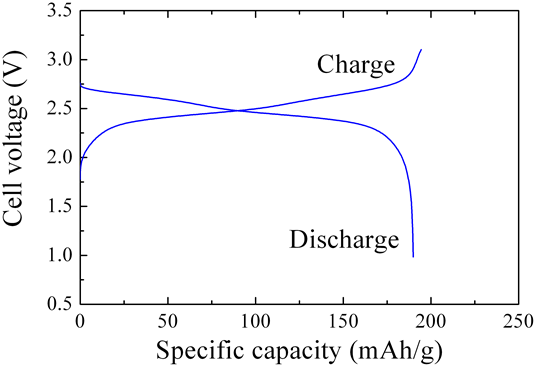
Figure 4 shows the results of an evaluation of the charge and discharge characteristics of a prototype lithium ion secondary battery using the newly developed titanium oxide as a negative electrode active substance, and lithium manganese oxide in the positive electrode. The results clearly indicate that the developed titanium oxide in this battery structure enables reversible charge and discharge, and that the material presents no issues when used in negative electrodes.
 |
|
Figure 4 : Charge-discharge characteristics of a prototype lithium ion secondary battery using new titanium oxide in negative electrode (Positive electrode: LiMn2O4; Negative electrode: H2Ti12O25) |
In future, ISK will distribute samples of the new material to the industry including battery manufacturers. Clarifying issues in relation to commercialization of the material, we will optimize its chemical composition, crystal structure, and powder characteristics, seeking in particular to improve its input-output characteristic.