Update(MM/DD/YYYY):10/27/2004
New High Capacity Li-Fe-Mn Positive electrode Materials Developed
- Opening The Way to Low Cost Lithium Ion Battery -
Key Points
-
New Fe-based oxide positive electrode materials with high capacity and superior high temperature cycle performance has been developed through the combination of chemical composition design fully utilizing ferric ion with novel synthetic route for getting nanometer-sized particles.
-
So far, none of iron-based positive electrode materials have been available, comparable to the existing ones (lithium cobalt oxide, lithium manganese spinel oxide) in respect to discharge voltage and capacity.
-
As iron resource is available abundantly, it will open the way to the development of low cost lithium ion battery.
-
The use of element in abounding resources will drastically expand the application of the Li-Fe-Mn positive electrode battery to the automobiles and other devices.
Synopsis
The Research Institute for Ubiquitous Energy Devices (RIUED) of the National Institute of Advanced Industrial Science and Technology (AIST), an independent administrative institution, has successfully developed new Li-Fe-Mn positive electrode materials lithium ion based secondary battery with 3 V or higher discharge voltage through the nano-particle synthetic route (wet chemical synthetic route) combining co-precipitation method with hydrothermal process, and demonstrated that the new positive electrode is superior to the conventional lithium-manganese-spinel oxide positive electrode in charge/discharge capacity for high temperature cycles; that it has approximately, 150 mAh/g capacity, comparable to that of lithium cobalt oxide, most widely used for existing lithium-ion battery and that the mean discharge voltage and energy density per mass of positive electrode materials may be upgraded through adjustment of chemical composition (adding nickel element), control of Fe ion arrangement, and improvement of preparation route (Fig. 1).
Heretofore, iron-based oxide, such as LiFeO2, has been regarded as a promising positive electrode material for lithium-ion battery. However, no positive electrode has been developed comparable to existing one in respect to discharge voltage and capacity, and no advantage has been demonstrated over the available one in charge-discharge properties.
Through the combination of chemical composition design fully utilizing ferric ion with the nano-particle synthetic route (wet chemical synthetic route) combining co-precipitation method with hydrothermal process, accumulated at the RIUED-AIST, the chemical composition and preparing conditions have been successfully optimized so as to meet two requirements simultaneously: reducing the primary particle size and suppressing ferric ion disordering. The new positive electrode has proved to have high capacity comparable to that of lithium cobalt oxide positive electrode, and better high temperature cycle characteristics than that made from lithium manganese spinel oxide.
The achievement is expected to open the way to commercial use of iron-based oxide positive electrode to lithium secondary battery, to reduce the cost of lithium ion battery and to ensure massive application of richly available positive electrode materials to automobile and other devices. The study has been carried out under a project (FY2002~2006) sponsored by the Ministry of Economy, Trade and Industry (METI) and the New Energy and Industrial Technology Development Organization (NEDO).
The study has been carried out under a project (FY2002~2006) sponsored by the Ministry of Economy, Trade and Industry (METI) and the New Energy and Industrial Technology Development Organization (NEDO). The further efforts will be paid to the upgrading of charge-discharge properties of the new materials, and to the reduction of manufacturing cost through simplifying preparatory conditions.
The further efforts will be paid to the upgrading of charge-discharge properties of the new materials, and to the reduction of manufacturing cost through simplifying preparatory conditions.
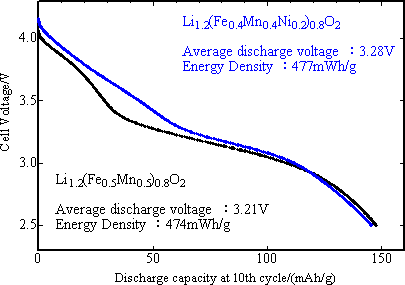 |
|
Fig. 1. Discharge curves of two newly developed positive electrode materials against carbon negative electrode after 10 cycles following charging up to 4.3 V at 60 °C. |
Background
The lithium-ion battery is an essential energy source for notebook PC, mobile phone and other devices, and lately, its application to larger-sized edition, such as vehicle batteries has been attempted. Among a number of constituents of lithium-ion battery, the most important ones affecting the battery performance are positive electrode materials, and R&D of transition metal oxide involving lithium ion, such as lithium cobalt oxide (LiCoO2), lithium nickel oxide (LiNiO2), lithium manganese spinel oxide (LiMn2O4) and so on, has been actively pursued. In most of existing lithium ion battery, lithium cobalt oxide is adopted. However, owing to recent rise in cobalt prices and requirements for battery cost reduction, it has been requested to reduce cobalt use in positive electrode. In particular, with massive use of positive electrode materials for large capacity lithium ion battery being intended for on-vehicle use, the development of positive electrode materials using richly available and less expensive transition metal oxide has been urgently demanded. While lithium manganese spinel oxide positive electrode material is most promising, its discharge capacity is 100 mAh/g which is less than that of lithium cobalt oxide, 150 mAh/g, and moreover, trivalent manganese dissolved out in electrolyte solution precipitates onto the carbon negative electrode to degrade the charge-discharge characteristics markedly, keeping intensive needs for the development of alternative materials.
Among candidate transition metals, iron is least expensive and most richly available The price of iron is about 1/100 that of cobalt and the amount of iron deposits is around 2,000 times as much as that of cobalt.[1] However, existing iron-based oxide positive electrode material such as LiFeO2 has average discharge voltage as low as 2 V or less, not adequate for alternative materials for existing positive electrode. Even with solid solution of iron in lithium cobalt oxide or lithium nickel oxide, the charge-discharge characteristics has not been improved appreciably, making the application of iron-based oxide to positive electrode materials nearly impossible.
[1] Yoshio, M. and Ozawa, A. (ed): “Lithium Ion Battery”, 2nd Edition, Materials and Application, p. 36, Nikkan Kogyo Shinbun, Tokyo----In Japanese.
History of R&D Work
For the purpose of developing ferric oxide-based positive electrode materials with charge-discharge capacity greater than those of lithium cobalt oxide, the study has been advanced on the basis of chemical composition design and precision control of transition metal ion distribution. In Fiscal Year 2001-02, positive electrode materials involving oxidation-reduction of ferric ion at 4 V region (iron-substituted Li2MnO3) has been found for the first time in the world.[2] However, the charge-discharge characteristics of resultant positive electrode materials were inferior to that of existing positive electrode, and further improvement of preparation conditions and chemical composition optimization has been needed. From FY2002, the R&D work has been supported by METI, and from FY2003 by NEDO. The result of the R&D work, characterized by the use of inexpensive and richly available constituent elements, mainly iron and manganese with around 20 % or less nickel, is expected to reduce the cost of positive electrode materials for lithium-ion battery and to save valuable resources.
[2] Journal of Power Sources, 97-98, 415-419 (2001); Journal of the Electrochemical Society, 149, A509-A524 (2002); and Patent #3500424.
Details of R&D Work
The newly developed iron-based oxide positive electrode materials consist of two kinds of compound with Li1.2M 0.8O2 composition, (where M = Fe 0.5Mn0.5, and Fe0.4Mn0.4Ni0.2), having the same layered rock salt structure as lithium cobalt oxide or lithium nickel oxide. These compounds are available in the form of nanometer-sized powder of primary particle size less than 100 nm, as shown in Fig. 2 and prepared through the process shown in Fig. 3. For improving the charge- discharge characteristics while fully utilizing ferric ion, it is necessary to suppress the disordering in ferric ion arrangement and to reduce the particle size. To achieve this, it is requested to prepare a homogeneous precursor in which transition metal ion uniformly distributed within the solid.
|
Li1.2(Fe0.4Mn0.4Ni0.2)0.8O2 |
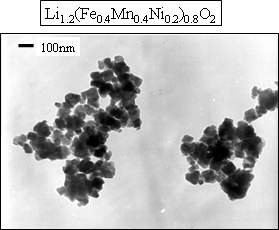 |
|
Fig. 2. TEM picture of newly developed iron-based positive electrode powder |
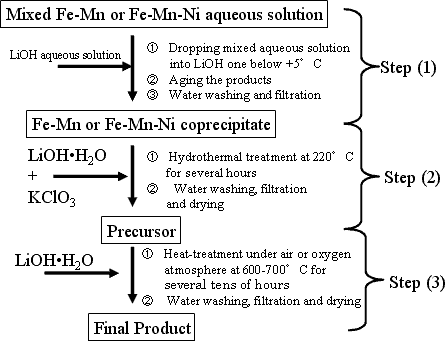 |
|
Fig. 3. Basic preparation process for newly developed iron-based positive electrode materials |
The formation of ferrite, which disturbs the electrode reaction, may be suppressed by controlling the co-precipitation temperature below +5°C in Step (1) and fast heating the starting mixture up to target temperature during hydrothermal reaction process in Step (2). The fine powdered and homogeneous nature of precursor result in the formation of homogeneous final products at relatively lower temperatures, 650 °C or lower due to its highly-reactive nature with lithium source.
In order to estimate exactly the discharge potential of positive electrode, the initial discharge characteristics was studied with the final product as positive electrode and metal lithium as negative electrode for 3 to 4.3 V potential range, current density 42 mA/g and at 30 °C (Fig. 4). The capacity of positive electrode material with M = Fe0.5Mn0.5 was 90 mAh/g or less, slightly lower than that of reference positive electrode of lithium manganese spinel oxide (LiMn2O4), while the capacity of nickel-substituted positive electrode was nearly same as that of the latter one, 100 mAh/g. The mean operating voltage was 3.8 V. Both capacity and voltage of the new positive electrode were highest among iron-based oxide positive electrodes. The newly developed positive electrode presented the charge-discharge characteristics comparable to that of lithium manganese spinel oxide. It has been reported that the lithium manganese spinel positive electrode has markedly degraded through the dissolution of Mn3+ ions in the charge-discharge cycle test at 60 °C with carbon used as negative electrode. In Fig. 5, the high temperature cycle performance of the newly developed positive electrode and that of lithium manganese spinel were compared using carbon negative electrode and under the same current density as in the test with metal lithium negative electrode and with the potential range of 2.5 ~ 4.3 V. While the capacity of lithium manganese spinel positive electrode was degraded after 10 cycles of operation, that of the newly developed positive electrode was not degraded, keeping the capacity close to about 150 mAh/g after 10 cycles. This is comparable to that of existing lithium cobalt oxide (150 mAh/g). This shows that the newly developed positive electrode has superior high temperature cycle characteristics to that of lithium manganese spinel.
The mean discharge voltage of Li
1.2(Fe
0.5Mn
0.5)
0.8O
2 positive electrode was 3.2 V, by about 0.4 V lower than that of lithium cobalt oxide, 3.6 V. Moreover, it was demonstrated in the similar experiment with the Li
1.2(Fe
0.4Mn
0.4Ni
0.2)
0.8O
2 positive electrode that the capacity was held unchanged after 10 cycles of operation, and that the mean operating voltage was raised by 0.07 V through the solid solution of 20% nickel ions. On the basis of these facts, it may be concluded that the newly developed positive electrode can be used as an alternative positive electrode materials for the existing positive electrode, but of different characteristics from that of manganese spinel oxide positive electrode.
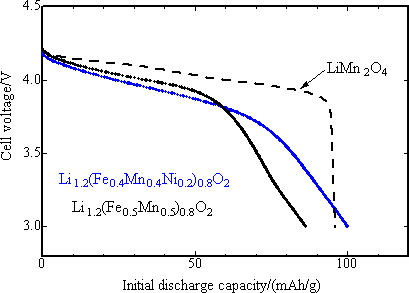 |
|
Fig. 4. The initial discharge characteristics of two varieties of newly developed iron-based positive electrode materials powder using metal lithium for negative electrode after charging up to 4.3 V, as compared with that of existing LiMn2O4 positive electrode materials. |
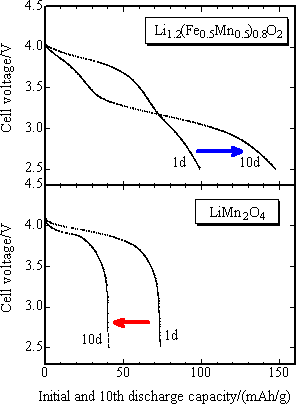 |
|
Fig. 5. The comparison of discharge curve of newly developed iron-based positive electrode materials (upper figure) with that of existing LiMn2O4 positive electrode (bottom figure) vs. carbon negative electrode, in the initial operation after charge-up to 4.3 V at 60 °C (1d) and after 10 cycles of operation (10d).The arrow shows change in discharge capacity after 10 cycles of operation |