The Correlated Electron Research Center (CERC) at the National Institute of Advanced Industrial Science and Technology (AIST) has developed organic crystals made from a single substance that are interchangeable (phase transition) between molecular crystal (neutral) and ionic crystal (ionic) states. The researchers proved the phase transition by (1) establishing a method to accurately reduce the phase transition temperature from room temperature to an extremely low temperature, (2) achieving for the first time a quantum tunneling state (quantum phase transition) between two states of different crystal-binding at absolute zero, and (3) observing this effect by measuring the optical spectra.
Using a single crystal of mixed-stack charge-transfer complex with DMTTF (4,4',5,5'-dimethyltetrathiafulvalene) as the electron donor, and QCl4 (ρ-Chloranil; see the chemical structure in Figure 1) and a bromine-substituted derivative as the acceptor, the team was able to apply pressure to accurately control the neutral-ionic phase transition temperature from absolute zero to room temperature (Figure 1). In the pressure ranges where phase transition begins to appear close to absolute zero, quantum paraelectricity was seen as proof that the electric polarization experiences a quantum-fluctuation effect. Moreover, the researchers were able to achieve almost the same method as the above pressure technique through a molecular size effect (effective pressure)—whereby the four Cl atoms on the QCl4 molecule were substituted one by one with Br atoms—showing accurate control to close to the point where the phase transition temperature disappears toward absolute zero (quantum critical point).
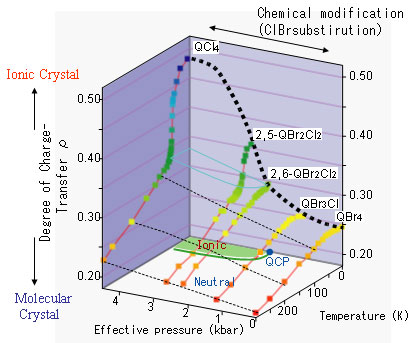
The team investigated thermal changes in the electric charge on the molecules in a series of the complexes, by measuring the optical spectra (Figure 2). For the 2,6-QBr2Cl2 (2,6-dibromo-3,5-dichloro-p-benzoquinone) complex in the vicinity of the quantum critical point, where the neutral-ionic phase transition occurs in response to effective pressure changes at the lowest temperature, the researchers observed the quantum paraelectricity as well as molecular charge fluctuation between the neutral-ionic states.
In summary, by reducing the phase transition temperature to close to absolute zero, the researchers achieved quantum fluctuation (tunneling) between the states of molecular and ionic crystals. As well as being of interest from the perspective of basic science, this phenomenon should be investigated further to explore as yet unknown characteristics and functionality in materials that undergo neutral-ionic phase transitions. We think the materials and control methods developed through this research may represent an important step forward.
The results of this research are published in the 10 January 2003 edition of the US journal Science.*
*Science, " Quantum Phase Transition in Organic Charge-Transfer Complexes" by Sachio Horiuchi, Yoichi Okimoto, Reiji Kumai, and Yoshinori Tokura
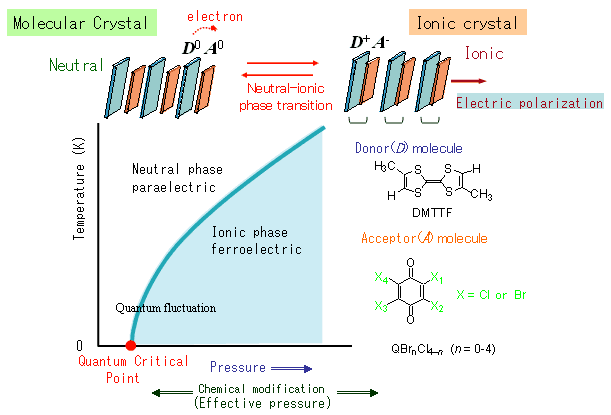 |
Figure 1. Temperature-pressure phase diagram and schematic diagram of the neutral-ionic phase transition in an organic mixed-stack charge-transfer crystal. |
 |
|
Figure 2. Changes of molecular charge ( ρfor D+ρA-ρ) in DMTTF-QBrnCl4-n crystals in response to temperature and effective pressure ( QBr4 used as the standard. Red line: thermal change of ρ for each complex; black line: variation of ρ as a function of effective pressure at a fixed temperature. The 2,6-QBr2Cl2 complex is closely located on the point of neutral-ionic transition at absolute zero, that is, on the quantum critical point (QCP). The bottom plane represents the neutral-ionic phase boundary. |