– Contributing to practical application of regenerative medicine with simple and rapid evaluation –
Hiroaki Tateno (Senior Researcher) and Jun Hirabayashi (Prime Senior Researcher), the Biotechnology Research Institute for Drug Discovery (BRD; Director: Masanao Oda), the National Institute of Advanced Industrial Science and Technology (AIST; President: Ryoji Chubachi), and Yuzuru Ito (Leader) and Yasuko Onuma (Senior Researcher), the Stem Cell Engineering Research Group, BRD, AIST, in collaboration with Hidenori Akutsu (Division Chief), the National Center for Child Health and Development (NCCHD), and Masashi Toyoda (Deputy Research Director), the Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology, have developed a technology to simply and rapidly evaluate the differentiation ability of human mesenchymal stem cells. Wako Pure Chemical Industries, Ltd. plans to commercialize this technology within one year.
Human mesenchymal stem cells have the capacity for self-renewal and differentiation, and since there is little risk of tumor formation, they have garnered attention as a cell source for regenerative medicine. However, there was no method for evaluating the differentiation ability of human mesenchymal stem cells, which posed a problem in applying them to regenerative medicine. The developed technology can evaluate the differentiation ability of human mesenchymal stem cells before transplantation, which is important for achieving the therapeutic effect, using specific lectins (α2-6 sialic acid-binding lectins). The technology is expected to contribute to the improved effectiveness of regenerative medicine using human mesenchymal stem cells.
These results were achieved in research projects commissioned by the New Energy and Industrial Technology Development Organization (NEDO) and the Japan Agency for Medical Research and Development (AMED). Details of this technology will be published online in an American academic journal, Glycobiology, on April 2, 2016 (Eastern Standard Time).
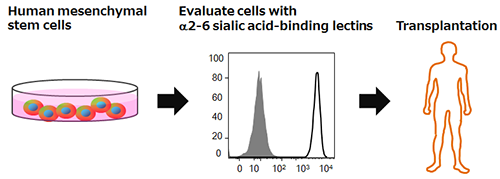 |
|
Summary of the technology for evaluating the differentiation ability of human mesenchymal stem cells |
Human mesenchymal stem cells are a type of somatic stem cell derived from the mesodermal tissue (mesenchyme) such as bone marrow and adipocytes, and have the capacity to differentiate into mesodermal tissues including osteoblasts, cartilage, and adipocytes, as well as to control the immune system. Therefore, human mesenchymal stem cells are expected as a cell source for regenerative medicine, and clinical testing has already been conducted for various diseases including graft-versus-host disease, which is a complication of organ transplants. While clinical application and commercialization of regenerative medicine using human mesenchymal stem cells progress, an issue has arisen concerning the quality of human mesenchymal stem cells. Mesenchymal stem cells taken from humans are transplanted for the treatment of various diseases after being grown and differentiated in vitro, but human mesenchymal stem cells are a heterogeneous cell group, and their nature changes depending on the donor, the culture conditions, and the number of subcultures. The differentiation ability of human mesenchymal stem cells is believed to have a large impact on the therapeutic effect of the cells, but until now there was no method for evaluating the ability. For this reason, there is demand for the development of technology that can simply evaluate the differentiation ability of human mesenchymal stem cells.
AIST has developed lectin microarrays, a technology that can analyze at high speed and with high sensitivity the sugar chains that densely cover cell surfaces. AIST also participates in projects commissioned by AMED since FY2015 (by NEDO in FY 2014) and implemented by the Stem Cell Evaluation Technology Research Association “Development of cell manufacturing and processing systems for commercialization of regenerative medicine/development of manufacturing systems for regenerative medicine products derived from human pluripotent stem cells (cardiac muscle, nerve, retinal pigment epithelium, and liver cells) and the development of manufacturing systems for regenerative medicine products derived from human mesenchymal stem cells”. Of these projects, the “Development of manufacturing systems for regenerative medicine products derived from human mesenchymal stem cells” project is advancing research and development aimed at achieving systems to manufacture and supply human mesenchymal stem cell products that reflect the needs of the clinical medical field to the maximum extent. The present research used lectin microarrays to work towards development of an evaluation technology for the differentiation ability of human mesenchymal stem cells.
Having analyzed various types of human mesenchymal stem cells using lectin microarrays, the researchers found that four types of α2-6 sialic acid-binding lectins (TJA1, SSA, SNA, rPSL1a) are highly reactive with human mesenchymal stem cells with strong ability to differentiate, and are less reactive with cells that have weak ability to differentiate.
After subculturing human adipose-derived mesenchymal stem cells for a long period of time, and inducing cells of early- and late-passage to differentiate into osteoblasts or adipocytes using culture solution for induction of differentiation, the cells were dyed with Alizarin Red S (dye that binds to calcium) and Oil Red O (dye that binds to lipid droplets) respectively. The results are shown in Fig. 1. Cells that differentiated into osteoblasts or adipocytes are both dyed red. Human adipose-derived mesenchymal stem cells from early-passage were dyed red after being induced to differentiate into osteoblasts or adipocytes, so it can be seen that they have the ability to differentiate into osteoblasts or adipocytes. Yet, while human adipose-derived mesenchymal stem cells from late-passage were induced to differentiate into osteoblasts or adipocytes, they were not dyed red, so it can be seen that they do not have the ability to differentiate into osteoblasts or adipocytes.
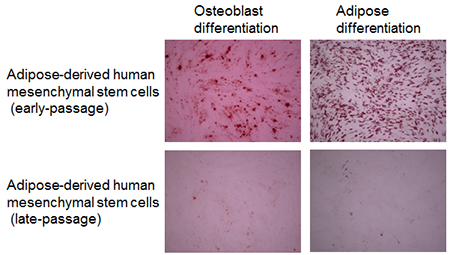 |
Figure 1: Differentiation into osteoblasts or adipocytes of early-passage and late-passage human adipose-derived mesenchymal stem cells
Osteoblast Differentiation: Dyed with Alizarin RED S (osteoblasts are dyed red.) Adipose Differentiation: Dyed with Oil RED O (adipocytes are dyed red.) |
Next, early-passage human adipose-derived mesenchymal stem cells that have the ability to differentiate and late-passage cells that do not have the ability to differentiate were analyzed with lectin microarrays. Four types of α2-6 sialic acid-binding lectins (TJA1, SSA, SNA, and rPSL1a) showed higher reactivity with the early-passage cells than with the late-passage cells. These four types of lectins were fluorescence-labeled, and their reactivity with the early-passage human adipose-derived mesenchymal stem cells that have the ability to differentiate and late-passage cells that do not have the ability to differentiate was analyzed using a flow cytometer (Fig. 2). The four types of lectins showed higher reactivity with the early-passage human adipose-derived mesenchymal stem cells having the ability to differentiate than with the late-passage cells, and as a result the early-passage cells were strongly fluorescent-dyed.
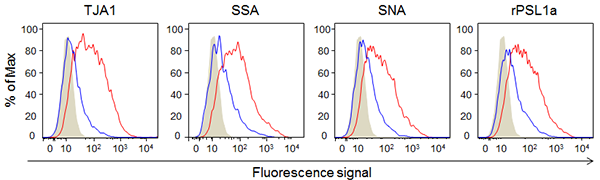 |
Figure 2: Reactivity of α2-6 sialic acid-binding lectins with early-passage and late-passage human adipose-derived mesenchymal stem cells
Gray: Control, Blue: Late-passage, Red: Early-passage
X-axis: Relative value of fluorescent intensity, Y-axis: Relative number of cells |
The binding form of sialic acid expressed in the cells was analyzed with liquid chromatography, and α2-6 sialic acid was detected in the early-passage human adipose-derived mesenchymal stem cells that have the ability to differentiate, but almost no α2-6 sialic acid could be detected in the late-passage cells that do not have the ability to differentiate. In human bone marrow-derived mesenchymal stem cells and cartilage-derived chondrocytes, compared with late-passage cells, early-passage cells with strong ability to differentiate were found to have higher reactivity with α2-6 sialic acid-binding lectins. On the other hand, human dermal fibroblasts that have almost no ability to differentiate had no reactivity with α2-6 sialic acid-binding lectins.
From these results, the researchers found that it is possible to evaluate the differentiation ability of human somatic stem cells such as human mesenchymal stem cells and chondrocytes by their reactivity with α2-6 sialic acid-binding lectins. Because this developed technology can easily evaluate the differentiation ability of human mesenchymal stem cells, it is expected to be applied to quality management in the manufacturing process of human mesenchymal stem cells used for regenerative medicine.
Out of the four types of α2-6 sialic acid-binding lectins, the fluorescence-labeled rPSL1a, a recombinant protein prepared in E. coli, is planned for commercialization within one year as a reagent for evaluating the differentiation ability of human mesenchymal stem cells. The researchers will verify its applicability to various types of human mesenchymal stem cells used in regenerative medicine, and contribute to the realization of regenerative medicine using human mesenchymal stem cells.