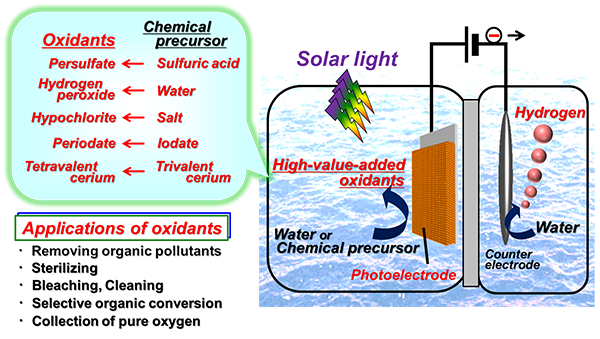
Kazuhiro Sayama (Prime Senior Researcher and Leader of Solar Light Energy Conversion Group) of the Energy Technology Research Institute (ETRI; Director: Haruhiko Obara), the National Institute of Advanced Industrial Science and Technology (AIST; President: Ryoji Chubachi), Kojiro Fuku (Researcher) and others of Solar Light Energy Conversion Group, ETRI, AIST, have developed a technology to produce hydrogen and various high-value-added chemicals efficiently and simultaneously using semiconductor photoelectrodes prepared by laminating porous tungsten trioxide (WO3) etc. for electrolysis of water which uses solar light energy (Fig. 1).
Oxidizing agents such as persulfuric acid, hypochlorite, hydrogen peroxide, periodate, and tetravalent cerium salt can be produced as the chemicals. In the reaction that converts and collects solar light energy into chemical energy as hydrogen and persulfuric acid, conversion into persulfuric acid with nearly 100 % selectivity and a very high level of solar light energy conversion efficiency (ABPE = 2.2 %) have been achieved. By using solar light energy, the technology makes it possible to reduce the voltage for water electrolysis significantly while producing hydrogen energy and various useful chemicals simultaneously. The technology also has the potential to realize new processes showing high economic efficiency in the future.
Detailed results will be presented at the 82nd Spring Meeting of the Electrochemical Society of Japan to be held on March 15-17, 2015, at Yokohama National University (Yokohama, Kanagawa Pref.).
 |
|
Figure 1: Production of hydrogen and high-value-added oxidizing agents using sunlight and a photoelectrode |
To build a sustainable society, it is essential to use renewable energy effectively, and particularly important to use the most abundant renewable energy, namely solar light energy. Artificial photosynthesis technology, which converts solar light energy directly into chemical energy and stores it in a process similar to plant photosynthesis, has been gaining attention in recent years. Many research projects are being conducted on the use of photoelectrodes and photocatalytic powders of oxides in addition to sunlight to synthesize oxygen and organic substances from water and carbon dioxide, and to synthesize hydrogen and oxygen from water (solar hydrogen production). If a solar hydrogen production system is developed that is as efficient as a solar cell and as simple and inexpensive as plant cultivation, it could potentially make a major contribution to realizing a hydrogen-based society and solving our energy problems. However, efficiency of photocatalysts and photoelectrodes to convert solar light energy into hydrogen energy, etc. is still low. A system having high performance and economic efficiency needs to be developed.
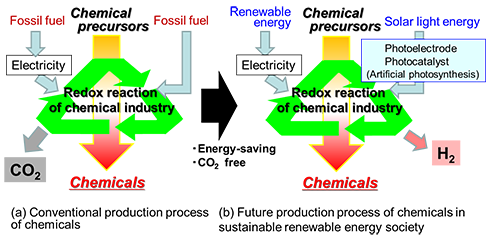
Moreover, production of various chemicals uses a massive amount of fossil fuel energy. Conserving energy in these processes and eliminating carbon dioxide emission are very urgent issues (Fig. 2). In most cases, the production of chemicals involves an oxidation-reduction reaction. If a photoelectrochemical process for producing chemicals by using solar light energy is achieved with high efficiency and low voltage, it could greatly lower energy consumption and costs. While this would be a major breakthrough for building a society based on renewable energy, this approach has not been extensively studied to date.
 |
|
Figure 2: Production of useful chemicals, now (a) and in the future (b) |
Previous AIST research has looked into the production of hydrogen from the electrolysis of water with porous photoelectrodes using various oxide semiconductors. In 2012, the world’s highest solar light energy conversion efficiency for the time (ABPE = 1.35 %) was achieved for production of hydrogen from the electrolysis of water using an oxide semiconductor photoelectrode (AIST press release on March 12, 2012). If the chemical reaction could be caused to proceed such that the oxidation-reduction potential was positively larger than the oxygen generation, it would reduce the energy loss and increase the conversion efficiency of solar light energy into hydrogen and oxidation products. Until now, however, more attention has been attracted to the production of hydrogen on the reduction side than to the reaction on the oxidation side. Therefore, there has been very little investigation into efficient production of high-value-added chemicals on the oxidation side, and the performance has been low.
This research was supported by “Japan-U.S. Cooperative Project of on Energy and Environmental Technology Research/Standardization (Japan-U.S. Environmental Technology Research Cooperation)” (FY2010-FY2014) of the Ministry of Economy, Trade and Industry.
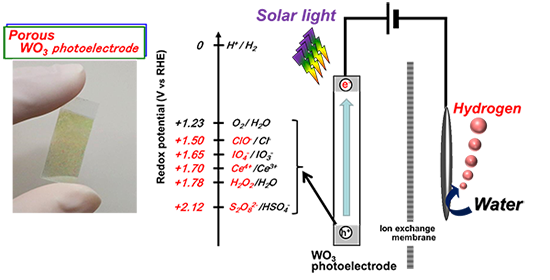
In this research, a semiconductor photoelectrode composed of a porous tungsten oxide film was prepared by a very simple method in which a solution containing tungstate ions was spin-coated on a conductive glass, and then the film was calcined. Production of hydrogen and high-value-added oxidants simultaneously was achieved with high efficiency by enhancing efficiency of light absorption and utilizing light scatter effectively using a thicker film (Figs. 1 and 3). An ion exchange film was placed between the photoelectrode and the counter electrode to prevent the reverse reaction. Various aqueous solutions containing oxidants were efficiently produced; the oxidants include persulfuric acid (S2O82-) from an aqueous solution of sulfuric acid (HSO4-), hypochlorite (ClO-) from an aqueous solution of sodium chloride (NaCl), hydrogen peroxide (H2O2) from an aqueous solution of carbonate, periodate (IO4-) from an aqueous solution containing iodate (IO3-), and tetravalent cerium salt (Ce4+) from an aqueous solution containing trivalent cerium salt (Ce3+). S2O82-, ClO-, and H2O2 were produced with the highest performance reported so far. Moreover, IO4- and Ce4+ are produced by novel reactions in this system. Potentials of all reactions are positively larger than the oxidation-reduction potential for generating oxygen (1.23 V(RHE)) and so these reactions can lead to the effective use of solar light energy. In particular, no oxygen generation was observed in the persulfuric acid production from an aqueous solution of sulfuric acid, and the selectivity to persulfuric acid as an oxidation product was almost 100 %. Generally, it is theoretically necessary to apply voltage of over 2.1 V to make this electrochemical reaction occur using conventional metallic electrodes. With the photoelectrode, however, the reaction can take place with as low as 0.6 V (Fig. 4). The solar light energy conversion efficiency (ABPE efficiency), which is an indicator of photoelectrode performance converting solar light energy into chemical energy of hydrogen and persulfuric acid with application of supplementary voltage, was 2.2 %. This value is the highest efficiency, and 1.6 times larger than past reported values. When the researchers used a platinum electrode instead of the photoelectrode for the oxidation reaction, only oxygen was generated; virtually no persulfuric acid was generated, even with voltage of 2 to 3 V supplied. This indicates that the developed semiconductor photoelectrode has excellent selectivity. It is presumed that a unique mechanism, namely, a direct reaction at the semiconductor’s positive holes, which have strong oxidation power, is involved, as shown in Fig. 3.
 |
|
Figure 3: Reaction mechanism for producing useful chemicals with photoelectrode |
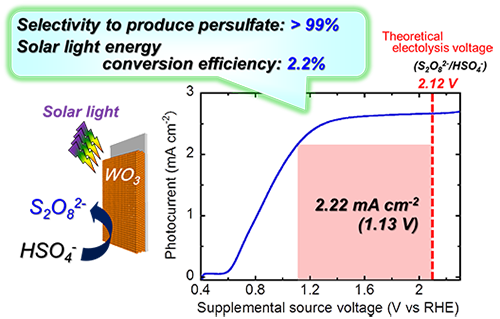 |
Figure 4: Current-voltage property of producing hydrogen and persulfuric acid with WO3 photoelectrode
(under solar simulator light) |
Hypochlorite is another useful oxidizing agent. In particular, it is widely used as bleach and disinfectant of drinking water. By using the WO3 semiconductor photoelectrode, the researchers were able to produce hypochlorite ions from a saline solution at a very low voltage of less than 1.1 V. They were even able to generate hypochlorite at low voltage by using a photoelectrode made up of bismuth vanadium oxide (BiVO4), an oxide semiconductor known to be more capable of using solar light energy than WO3. The selectivity of product to photocurrent was about 46 %. Currently, hypochlorite is produced in large quantities using a massive amount of electric energy (an electrolysis voltage of 1.4 V or higher) through direct or indirect electrolysis of saline solution. If a more efficient production of hypochlorite is achieved at lower voltage using the photoelectrode and solar light energy, a significant energy-saving effect could be expected.
Furthermore, the researchers found that when using an aqueous solution of carbonate with these WO3 or BiVO4 photoelectrodes, hydrogen peroxide was generated by oxidation of water. The selectivity of product to photocurrent was about 49 %. The hydrogen peroxide, which also possess strong oxidation power and produces only water after utilization, has been widely utilized as a clean and versatile oxidant. Hydrogen peroxide is produced primarily with the anthraquinone process, however many issues result. For example, the production process is complex and uses a large volume of organic solvents. In addition, the selectivity of products for generating IO4- and Ce4+ in the novel reactions was about 50 % and 40-50 %, respectively with the WO3 photoelectrode.
The various oxidizing agents produced in this study can be used in various fields by their strong oxidation power. Applications could include removal of organic pollutants, effluent treatment, bleaching, sterilizing, cleaning agents and selective organic transformation. If the oxidizing agents in the aqueous solution were decomposed in a separate location, it would also be possible to produce oxygen of high purity, making it easy to collect only pure oxygen gas. Also, after these oxidizing agents are used, they return to their original raw materials. The system presented here is powered by inexhaustible solar light energy and offers a way to produce and store useful chemical agents and hydrogen energy simultaneously in water, a clean solvent. The system is a ground-breaking outcome that demonstrates the future potential of innovative and effective ways to use solar light energy.
To enhance the solar light energy conversion efficiency of photoelectrodes, it is necessary to enhance photocurrent and selectivity, and decrease supplementary power source voltage. It has already been confirmed that it is possible to produce high-value-added chemicals by using photoelectrodes of oxide and non-oxide semiconductors, etc. with greater potential for a wide range use of sunlight than is the case with the WO3 and BiVO4 semiconductors. Currently, the researchers are investigating how to enhance the performance of these photoelectrodes.
Research is also proceeding on an all-in-one independent system using inexpensive organic photovoltaic cells as a supplementary power source for the photoelectrode. The research seeks to achieve such things as hydrogen production that is even less costly than simple solar cells with water electrolysis and the creation of a drinking water purification system that is more energy efficient, through optimizing the overall system and making it even more efficient.