Yuichiro Himeda (Senior Researcher) and others, Solar Light Energy Conversion Group, the Energy Technology Research Institute (Director: Yasuo Hasegawa) of the National Institute of Advanced Industrial Science and Technology (AIST; President: Tamotsu Nomakuchi), have developed a highly efficient catalyst for the interconversion of carbon dioxide and formic acid in collaboration with Etsuko Fujita (Senior Chemist), Brookhaven National Laboratory (BNL) in the U.S.A. The catalyst can be used to produce formic acid (HCO2H) by the reaction of hydrogen with carbon dioxide (CO2) in water at ambient temperature and pressure. Conversely, carbon monoxide (CO)-free high-pressure hydrogen gas suitable for use in polymer electrolyte fuel cells can be supplied by the decomposition of formic acid.
An AIST - BNL collaborative research project based on Japan - USA Cooperation on Clean Energy Technologies has made this technology possible by developing new design principles for ligands that activate catalysts and hydrogen molecules. This catalyst technology can substantially improve the energy efficiency of the interconversion of carbon dioxide and formic acid and is expected to lead in future to the development of a large system for hydrogen storage using carbon dioxide.
Details of the results will be published in the electronic version of a British scientific journal, Nature Chemistry, on March 19, 2012 (JST).
 |
|
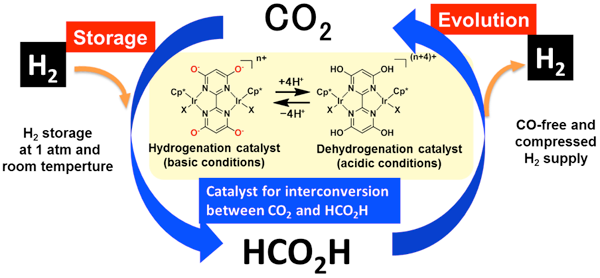
Highly efficient interconversion of carbon dioxide and formic acid by using a proton-responsive catalyst and a proton-relay catalyst |
To reduce carbon dioxide emissions and build a sustainable society, one goal is to create a hydrogen-energy society based on hydrogen as a clean energy source. Technology to safely and efficiently store and transport hydrogen that has low energy density is essential to achieve the goal. Development of a technology to reduce carbon dioxide and store hydrogen (energy), as in the dark reaction of photosynthesis, would help to realize a hydrogen society and lead to beneficial uses of carbon dioxide. Methanol and formic acid produced by the hydrogenation of carbon dioxide are liquid at room temperature and have relatively high energy densities. Therefore the research and development of them as easy-to-store and easy-to-transport hydrogen-storage materials has been conducted. However, there have been some issues with the materials: (1) the carbon dioxide conversion process requires high-temperature and -pressure conditions and is energy-intensive; (2) the concentration of carbon monoxide, which poisons the fuel cell electrode, needs to be kept below 10 ppm in the fuel cell to use the hydrogen regenerated by the reverse reaction process; and (3) the released hydrogen needs to be pressurized to supply it to the fuel cell. There has therefore been a need to develop a high-performance catalyst that substantially improves the energy efficiency of the conversion reactions in the storage and release of hydrogen.
AIST has been researching the production of formic acid by the hydrogenation of carbon dioxide and the production of hydrogen by the decomposition of formic acid. It has found one of the world’s highest-performance catalysts activated by a proton-responsive ligand and has succeeded, for the first time in the world, in producing formic acid from carbon dioxide and hydrogen in water at ambient temperature and pressure. It has also succeeded in the world’s most efficient release of CO-free hydrogen by decomposing formic acid in water containing no organic additives at a temperature below 100 °C. AIST has advanced catalyst development technology for both hydrogen storage (formic acid production) and hydrogen release (formic acid decomposition) processes, whereas BNL has technologies for analyzing the reaction mechanisms of artificial photosynthetic catalysts and for hydrogen activation modeled on a proton relay. The researchers have developed a high-performance catalyst in accordance with new catalyst design principles based on a combination of catalyst technologies from both AIST and BNL.
This research and development was supported by the Japan - USA Cooperative Project on Research and Standardization of Energy and Environmental Technologies of the Ministry of Economy, Trade, and Industry and the project is based on an agreement reached at the Japan - USA Summit Meeting of November 13, 2009 on Japan - USA Cooperation on Clean Energy Technologies.
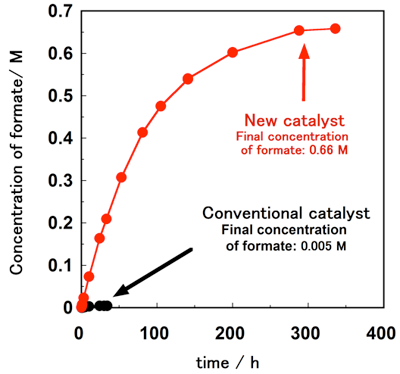
The conventional proton-responsive catalyst developed by AIST can produce formate by the hydrogenation of carbon dioxide in water at ambient temperature and pressure, but the reaction rate and yield (the amount of hydrogen stored) were not satisfactory (Fig. 1). The newly designed and synthesized catalyst has proton-responsive hydroxyl groups (-OH) placed near iridium atoms and activates hydrogen molecules through the interaction with the iridium atom and the oxygen atom in the hydroxyl groups. When carbon dioxide is hydrogenated in water at ambient temperature and pressure using this catalyst, the hydrogenation rate is more than 10 times that achieved with the conventional catalyst. The concentration of formate (the amount of hydrogen stored) is more than 100 times higher. In other words, use of the new catalyst has made it possible to convert hydrogen into formate at ambient temperature and pressure, instead of at energy-intensive, high-temperature and -pressure conditions.
 |
|
Figure 1 : Time course of production of formate by hydrogenation of carbon dioxide in water at ambient temperature and pressure |
Hydrogen containing no carbon monoxide is released through the decomposition of formic acid by the conventional proton-responsive catalyst under relatively mild conditions and without the use of organic additives. However, for the practical supply and use of hydrogen, it was necessary to increase the reaction rate and to compress (i.e. pressurize) the hydrogen gas released. The developed catalyst increases the reaction rate by more than 10 times (Fig. 2). When formic acid is dehydrogenated in a closed reaction vessel, the reaction rate does not decrease and formic acid is almost completely decomposed with no carbon monoxide produced as a by-product. As hydrogen is produced, the pressure in the closed reaction vessel increases, allowing the supply of high-pressure hydrogen without the use of an external pump.
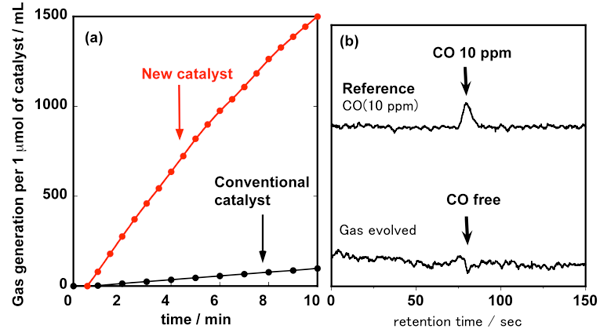 |
Figure 2 : (a): Time course of the amount of the evolved gas by the decomposition of formic acid
using the new catalyst and the conventional catalyst (dehydrogenation temperature: 90 °C)
(b): Results of analysis of the evolved gas by the decomposition of formic acid |
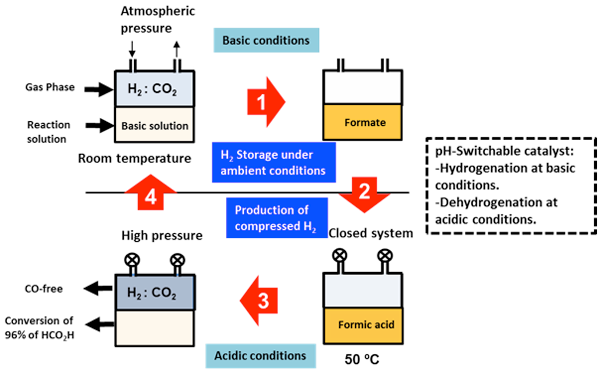
Figure 3 is a flow chart of a reversible and recyclable hydrogen storage system using the developed catalyst. Hydrogen gas is stored as 0.48 M formate by blowing a mixture of carbon dioxide and hydrogen (1:1 ratio) into an alkaline solution with the dissolved catalyst at ambient temperature and pressure (Step 1). Then, the pH of the reaction solution is adjusted to 1.7 by adding an acid (Step 2). The performance of the proton-responsive catalyst changes with the change in pH. When the reaction vessel is closed and heated to 50 °C, the formic acid is decomposed and compressed hydrogen gas is produced (Step 3). At the end of the reaction, more than 96% of the formic acid has been decomposed. When a base is added to the solution again (Step 4), hydrogen can be stored. Thus, the process of storing hydrogen at ambient temperature and pressure and releasing high-pressure hydrogen is repeated. It is not necessary to add more catalyst.
 |
|
Figure 3 : Flow chart of the hydrogen storage process based on a combination of hydrogenation of carbon dioxide and decomposition of formic acid |
This catalyst technology has made it possible to produce formic acid (hydrogen storage) by the hydrogenation of carbon dioxide and to supply high-pressure hydrogen gas by the decomposition of formic acid under mild conditions. Since the energy change associated with the interconversion reaction of carbon dioxide and formic acid is smaller than those associated with conversion reactions of methanol and other hydrocarbon-based hydrogen storage materials, catalysts have the potential to dramatically improve the energy efficiency of the interconversion reaction. With the development of higher performance catalysts it should be possible to develop a large and affordable hydrogen storage system using carbon dioxide.
Using the ability of the catalyst to supply high-pressure gas, the researchers are developing a high-purity hydrogen production system based on the continuous decomposition of formic acid combined with a carbon dioxide separation system. Using the new catalyst design principles, they intend to further increase the efficiency of the catalyst and reduce its cost. In addition, they aim to develop a completely artificial photosynthetic system to produce an energy storage material from water and carbon dioxide by combining the hydrogenation of formic acid with hydrogen production by solar energy using a visible light-responsive semiconductor catalyst.