- An innovative high-capacity, low-cost storage battery using metals as positive and negative electrodes -
Haoshen Zhou (Leader), the Energy Interface Technology Group, the Energy Technology Research Institute (Director: Yasuo Hasegawa) of the National Institute of Advanced Industrial Science and Technology (AIST) (President: Tamotsu Nomakuchi), and Yonggang Wang (Japan Society for the Promotion of Science (JSPS) Postdoctoral Fellow), have succeeded in developing an easily recyclable high-capacity “lithium-copper rechargeable battery.”
Lithium-ion batteries are widely used in cellular phones and notebook PCs. Recently, extensive research has been conducted on the development of high-capacity lithium-ion batteries for electric vehicles. Owing to limited lithium resources, the development of inexpensive and recyclable lithium-ion batteries is expected.
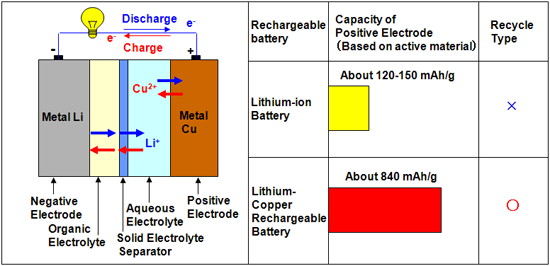
In our lithium-copper rechargeable battery, the negative electrode made of metallic lithium is dipped into an organic electrolyte solution, and the positive electrode made of metallic copper is dipped into an aqueous electrolyte solution. Mixing of the two electrolyte solutions is prevented by using a solid electrolyte separator. Lithium ions (Li+) can pass through the separator, while other ions (Cu2+, H+, OH-, etc.) cannot migrate from the aqueous electrolyte to the organic electrolyte; hence, the battery reactions proceed smoothly. The discharge capacity density of this battery is 843 mAh/g (per unit weight of copper consumed in the positive electrode reaction), which is more than five times the capacity of positive electrode in conventional lithium-ion batteries. The discharge capacity decreases only slightly even after 100 charge/discharge cycles. Recycling of conventional lithium-ion batteries, which contain electrodes having complex structures, is extremely difficult. However, the electrodes in our lithium-copper battery are made of metallic lithium and copper and have simple chemical reaction concept and electrode structures; hence, the manufacturing cost of this battery is low, and it can be easily recycled.
The results of this research will be presented at Scalable Energy Storage, which is sponsored by IBM, held in San Jose, USA, on August 26 and 27, 2009.
|
|
(left): Schematic illustration of the novel “lithium-copper rechargeable battery”
(right): Comparison of performances of the lithium-copper battery and a conventional lithium-ion battery
Unit: Discharge capacity per unit weight of active material used in the positive electrode (mAh/g) |
Lithium-ion batteries are widely used in cellular phones and notebook PCs, and extensive research is being carried out on the development of high-capacity batteries that can be used in electric vehicles. However, in the conventional lithium-ion batteries, the positive electrode contains cobalt or manganese, whose resources are limited. Further, when manufacturing these batteries, complex and expensive processes such as high-temperature sintering and control of fine structures are necessary. In addition, when recycling dead batteries (after hundreds or thousands of charge/discharge cycles), it is difficult to separate the active material from conductive additives, carbon, binder and the collector electrode. To overcome these problems, it is expected to develop inexpensive, high-capacity lithium-ion batteries that can be easily recycled.
Research carried out on the development of next-generation lithium-ion batteries at the Energy Technology Research Institute, AIST, showed that nano-structuring of electrode materials helps increase the power density of these batteries (AIST press releases on January 18, 2005; November 19, 2007; and August 27, 2008). Extensive studies have also been carried out on the development of “lithium-air batteries” with enhanced energy density for use in electric vehicles (AIST press release on February 24, 2009). Currently, researchers at AIST are focusing on the development of high-capacity lithium-ion batteries that can be easily recycled.
This research was supported in part by the Grants-in-Aid for Scientific Research of JSPS.
In this study, we have developed a rechargeable battery in which organic and aqueous electrolyte solutions are used on the negative electrode (metallic lithium) and positive electrode (metallic copper) sides, respectively; the two electrolyte solutions are partitioned by a solid electrolyte separator to prevent them from mixing. The electrolyte separator allows only lithium ions (Li+) to pass to the positive electrode side, but does not allow any other ions (Cu2+, H+, OH-, etc.) to migrate to the organic electrolyte on the negative electrode side; hence, stable battery reactions (charging/discharging) are realized.
During charging, the following reactions occur at the electrodes:
1) Negative electrode: Li
+ + e
- → Li
Lithium ions (Li+) from the aqueous electrolyte solution on the positive electrode side pass through the solid electrolyte separator and reach the surface of the negative electrode; here, they are supplied with the electrons from the external circuit and precipitate as metallic lithium (plating).
2) Positive electrode: Cu → Cu
2+ + 2e
-
Metallic copper dissolves in the aqueous electrolyte solution as copper ions (Cu2+), and electrons are released to the external circuit.
The following reactions occur during discharging:
1) Negative electrode: Li → Li
+ + e
-
Metallic lithium dissolves as lithium ions (Li+) in the organic electrolyte solution, and electrons are released to the external circuit. The lithium ions then migrate to the aqueous electrolyte solution on the positive electrode side through the solid electrolyte separator.
2)Positive electrode: Cu
2+ + 2e
- → Cu
The copper ions (Cu2+) migrating from the aqueous electrolyte solution to the surface of the positive electrode precipitates as metallic copper with the electrons supplied from the external circuit.
|
|
|
|
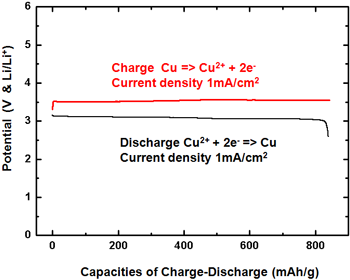
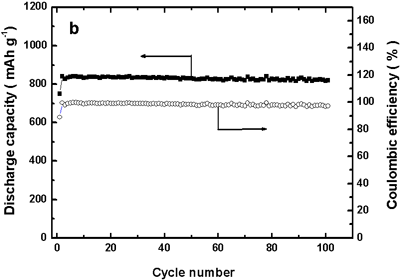
Figure 1. Charge/discharge curves (left) and charge/discharge cycle performance properties (right) of the developed battery |
The density of the discharge capacity of the positive electrode in this lithium-copper battery is around 843 mAh/g (per gram of copper reacting on the positive electrode). This discharge capacity is substantially larger than that of the positive electrode of a conventional lithium-ion battery (120–150 mAh/g, a literature value). From Fig. 1 (left), it is apparent that the discharge capacity of our battery decreases only slightly even after 100 charge/discharge cycles.
The electrodes in the lithium-copper rechargeable battery are constructed from pure metals and have simple structures. During charging and discharging, dissolution of the metal ions and precipitation (plating) of the metal occur. Therefore, the active electrode materials remain in metallic form after use, and this enables easy recycling of the materials (collection and recovery of active materials). Besides, since the aqueous and organic electrolyte solutions are divided by the solid electrolyte separator, each electrolyte can be recovered separately. Thus, recycling of these batteries is markedly easier than that of conventional lithium-ion batteries, because the recycling cost of the novel battery is expected to be very low.
The charging/discharging reactions occurring in this battery involve dissolution of metal ions on the metal surface and ”plating” of the metal electrode surface, and no complex processes such as compound formation occur; therefore, the current density of charge/discharge in this battery is expected to be very high. However, the lithium ion conductivity of the solid electrolyte separator is not sufficiently high, and hence, further research is necessary for enhancing the power density.
Although the discharge capacity density of the lithium-copper rechargeable battery developed in this study is much larger than that of conventional lithium-ion batteries, its power density must be improved so that it can be used in electric vehicles that consume a large amount of power. This will be possible if the lithium ion conductivity of the solid electrolyte separator is increased. AIST is planning to carry out further research for increasing the power density of this lithium-copper rechargeable battery.