- Cadmium-free and enables dynamic observation of a wide range of biological substances -
Norio Murase (Senior Research Scientist) and his colleagues of the Nano-structured Photonic Device Group (Leader: Junji Nishii), the Photonics Research Institute (Director: Masanobu Watanabe), the National Institute of Advanced Industrial Science and Technology (AIST) (President: Hiroyuki Yoshikawa), have developed indium phosphide (InP) photoluminescent NCs. These NCs are dispersible in water and stable for a long period and they exhibit high PL efficiency (68% in case of red).
The NCs have an InP/zinc sulfide (ZnS) core-shell structure, where InP is the core and ZnS is the coating on the outside. By controlling the reaction conditions, the ZnS shell was thickened and improved PL efficiency and chemical stability were achieved. At the same time, sulfur-containing surfactant was bound on the NC surface to increase water dispersibility, which was essential for biological applications.
Conventionally, cadmium selenide (CdSe) with ZnS shell, cadmium telluride (CdTe) with cadmium sulfide (CdS) shell, etc., have been used as photoluminescent NCs for the in vivo observation of quantity, distribution, and movement of trace substances such as in cultured cells. Although these NCs can be dispersed in water, their range of application is limited because cadmium causes cell death.
The developed NCs are expected to be used in wider applications when compared to the conventional cadmium-containing NCs.
This technique will be demonstrated in “nano tech 2009” to be held at Tokyo Big Sight from February 18 to 20, 2009.
 |
|
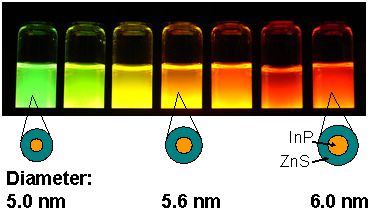
PL of the developed water-dispersible NCs under UV irradiation. The PL color changes in accordance with the particle size of the InP (core).
|
There is an increasing demand for high brightness of stable fluorescent reagents that are used for in vivo and in vitro biological studies to bind to biological substances so that the quantity, distribution, and movement of the substances can be examined. Semiconductor NCs with diameters of approximately 2 - 6 nm (nanometers) emit light with high efficiency when their surface conditions are well-controlled. CdSe and CdTe have been conventionally used as semiconductor NCs. It is possible to improve the precision of light emission detection since CdSe and CdTe have high brightness and their absorption and emission wavelength regions are far apart from each other. Furthermore, light is emitted in various different colors with a single wavelength excitation light by using NCs of various sizes since the emitted wavelength varies in accordance with the size of the NC due to the quantum size effect.
However, it has been reported that cadmium-containing NCs cause cell death in tests that use cultured cells. Although it is possible to reduce the level of dissolution by contriving the NC coating, it is more effective to exclude cadmium.
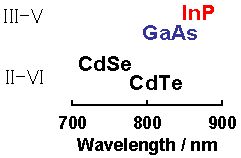
The emission wavelength of semiconductor NCs becomes shorter than that of the bulk bodies due to the quantum size effect. A semiconductor, whose bulk body emission wavelength is longer than the wavelength of red light, is required in order to obtain NCs that emit green to red light. The types of such group II–VI and group III–V semiconductors and the emission wavelengths of their bulk bodies are shown in Fig. 1.
 |
|
Figure 1. Types of group III–V and group II–VI semiconductors that emit light in long wavelength region and their emission wavelengths (bulk bodies)
|
According to Fig. 1, it is evident that the only candidate that does not contain cadmium (Cd) or arsenic (As) and emits light with a long wavelength is InP. Several attempts have been made in various regions of the world, including Germany and the U.S., to prepare InP NCs. However, their preparation requires severe reaction conditions and their emission intensity decreases when they are suspended in water. Therefore, our objective was to prepare the NCs by a safe and simple method while delivering high PL efficiency even in water.
This study was supported in part by the Creation and Support Program for Start-ups from Universities, Project to develop “Innovative Seeds”, sponsored by the Japan Science and Technology Agency (JST).
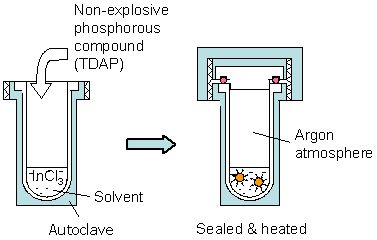
InP NCs (diameter: 2.3 - 4.0 nm) were synthesized within an organic solvent at relatively low temperatures (150 - 180°C) using tris(dimethylamino)phosphine (TDAP, [(CH3)2N]3P), a non-explosive phosphorous compound, by the solvothermal synthesis, as shown in Fig. 2.
 |
|
Figure 2. Preparation of InP NCs by the solvothermal synthesis
|
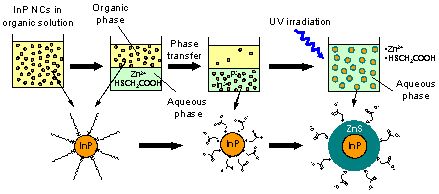
Next, we found that InP NCs move into the aqueous phase when they are brought into contact with the alkaline aqueous phase that dissolves sulfur-containing surfactant (thioglycolic acid (TGA)) and zinc ion, as shown in Fig. 3. Since the In-P bonding has large covalent character, InP has poor reactivity and dissolves only in aqua regia. However, we found that its reactivity increases by making it into NCs and that these NCs slightly dissolve and move into the aqueous phase. When UV light is irradiated on the NCs, TGA is decomposed by the photochemical reaction on the NC surface and a protective ZnS shell is formed.
 |
|
Figure 3. Method for the preparation of water dispersible high PL efficiency InP/ZnS NCs comprising of three stages: synthesis in organic solvent, transfer into aqueous phase, and light irradiation in the aqueous phase
|
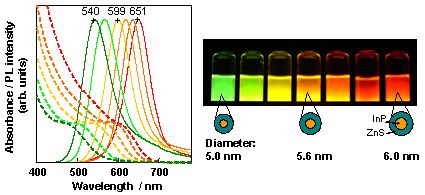
We found that a core-shell-type NC with InP core and thick ZnS shell can be prepared by these three-stage reactions. Since this NC surface is coated by a surfactant, the NCs disperse well in water and emits light with high PL efficiency (30% or higher with green to red wavelength region; 68% at maximum (red)). Figure 4 shows the absorption and emission spectra and an appearance of PL under UV irradiation.
 |
|
Figure 4. Absorption (broken line) and emission (solid line) spectra for InP/ZnS NCs dispersed in water and their PL under UV irradiation
|
Conventionally, PL efficiency was increased by coating ZnS shell on CdSe NCs. Zn and S were formed by pyrolysis in an organic solvent to allow the epitaxial growth of ZnS layer on CdSe surface. Therefore, it is known that the layer peels off due to the difference in lattice constants when one tries to add a thicker shell. Furthermore, in the case of CdSe/ZnS, the exciton little spreads over the NCs and ZnS shell with a thickness of approximately 0.5 nm is sufficient to confine exiton in the NCs.
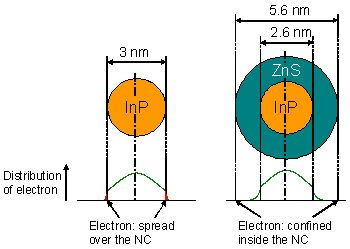
On the basis on this fact, ZnS shell was formed on the InP NCs by the epitaxial method in an organic solvent. However, sufficiently high PL efficiency was not obtained. In this study, we triggered the chemical reaction on the surface by irradiating UV light after transferring the InP NCs into the aqueous phase, and successfully formed a thick ZnS shell (1.5 nm). As shown in Fig. 5, it was indicated by calculation that this thick shell was necessary in order to confine the exciton in this NC as well.
This ZnS shell not only increases the PL efficiency but also improves the chemical stability of the NCs; thus, it is advantageous when they are used in the environment containing high concentrations of salts and so forth as is often in the case of biological fields. The PL efficiency and, emission and absorption spectra of the NCs did not change after several months in storage in the atmospheric air at room temperature.
 |
|
Figure 5. Relationship between the wave function of electrons of exiton in NCs and the ZnS shell thickness of InP/ZnS NCs
|
As a consequence of composition analysis, it was found that the molar ratio of group III (In) against group V (P) (number of group III atoms/number of group V atoms) was larger (approximately 1.8) than the conventionally reported value (approximately 1.1) for the developed InP/ZnS NCs. This indicates that the NCs prepared by this method have more In atoms allocated on the surface.
Practically, according to the periodic table, the only binary semiconductor material with green and red PL and without strong toxicity such as cadmium is InP. Since we can deliver high and stable PL efficiency in water by the safe preparation method of this InP, it is of a large practical significance. In the future, we plan to examine the mass productivity of the NCs, prepare for establishment of venture business and cooperate with related manufacturers so that InP can be used in a wide range of applications in the field of biotechnology.