Update(MM/DD/YYYY):10/16/2007
Development of a Fluorescent Organic Nanotube
- Useful for analysis of in vivo behavior of organic nanotubes -
Points
-
Fluorescent organic nanotubes are able to be manufactured by mixing fluorescent molecules with tube-forming amphiphiles during a manufacturing process.
-
Succeeds in brightening organic nanotubes in four colors.
-
Offers the possibility to observe transport of organic nanotubes filled with a drug within an organism.
Summary
Masumi Asakawa (Senior Research Scientist) of the High-Axial-Ratio Nanostructure Fabrication Team, the Nanoarchitectonics Research Center (Director: Toshimi Shimizu) of the National Institute of Advanced Industrial Science and Technology (AIST) (President: Hiroyuki Yoshikawa) developed organic nanotubes that generate fluorescence (fluorescent ONT-AIST) by embedding fluorescent molecules in the membrane wall of organic nanotube (organic nanotube AISTTM, ONT-AIST), which are formed through self-assembly of molecules. (Figure 1)
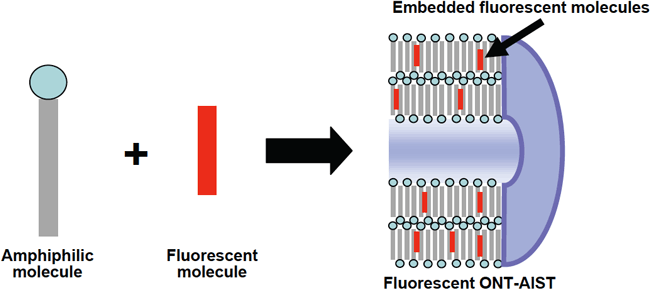
The newly-developed fluorescent ONT-AIST is created by adding fluorescent molecules to amphiphilic molecules during self-assembly process of the nanotubes in solution. The fluorescent molecules are stably embedded in the wall of nanotubes. The tubular structure of the ONT-AIST remains hollow even after fluorescent molecules are added, and the fluorescent ONT-AIST still has the ability to include molecules such as drugs inside the hollow cylinder.
Fluorescent ONT-AIST allows in vivo observation of ONT-AIST previously impossible, and offers great potential for analysis of transport of drug by ONT-AIST.
Part of these research results will be exhibited at Bio Japan 2007, to be held in Pacifico Yokohama from 19th to 21st of September.
 |
|
Fig. 1: The photograph of the organic nanotubes in non fluorescence, red, orange, yellow and blue color fluorescence.
|
Social Background of Development
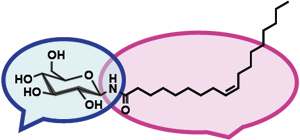
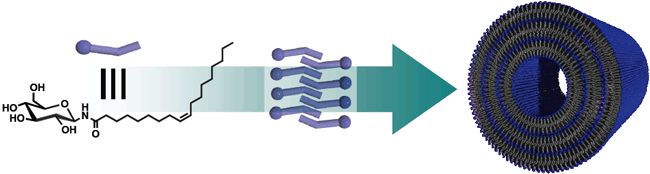
Amphiphilic molecules synthesized from glucose and oleic acid contained abundantly in olive oil, form a tubular structure through self-assembly to create ONT-AIST with excellent dispersibility in water and the ability to include protein and nucleic acid. These properties indicate potential for use in applications in wide range of fields such as medicine, health and food products. (Figure 2)
As anticancer drugs used for chemotherapy are toxic to normal healthy cells, side effects are unavoidable when trying to destroy cancer cells. To solve this problem, research is under way to develop a drug delivery system (DDS) that transports a drug only to cancer cells. ONT-AIST has a tubular structure with an opening at both ends, and potential as a new material for DDS able to release an agent gradually from both sides.
However, it is necessary to observe drug inclusion and transport of ONT-AIST in vivo for performance evaluation. ONT-AIST has not yet been put to practical use due to difficulty in in vivo observation.
 |
 |
|
Glucose: The energy source for the brain and body to move. It is produced the sugar of the food being digested.
4Kg / ¥8,500
|
Oleic acid: It decreases cholesterol. It is included in olive oil abundantly.
500ml / ¥1,400
|
ONT-AIST white solid powder (weight 140 g) (right) and the scanning electron microscope image (average outer diameter = 300 nm, the average inner diameter = 90 nm) (left)
|
 |
|
Fig. 2: ONT-AIST which can be synthesized in large quantities by self-assembly of amphiphilic molecules.
|
History of Research
The Nanoarchitectonics Research Center of AIST has been studying the molecular design, synthesis, and self-assembly of amphiphilic molecules to create organic nanotubes over the past 10 years. The research center successfully developed mass synthesis technology for ONT-AIST in 2006, and is currently developing applications for ONT-AIST.
Research on DDS using liposomes is being vigorously pursued. To observe liposomes within an organism, fluorescent molecules are usually chemically bonded to liposomes (fluorescent labeling) or expensive fluorescent labeling agents are used. Changing the color of fluorescent liposomes takes time and is costly, because the fluorescent labeling with different fluorescent molecule is needed for each color.
To solve this problem, AIST attempted to develop a simplified manufacturing method for fluorescent nanotubes to facilitate in vivo observation of ONT-AIST without losing its ability for inclusion or transport.
This research was conducted as part of “Solution Oriented Research for Science and Technology (SORST)” project of the Japan Science and Technology Agency (JST) from 2005 to 2008.
Details of Research
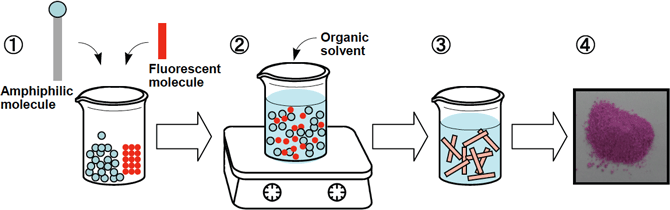
Standard fluorescent molecules were used to enable ONT-AIST to generate fluorescence. If a fluorescent molecule is added to amphiphilic molecules during the self-assembly process in an organic solution used in the ONT-AIST mass synthesis method developed by AIST, the fluorescent molecules are embedded in the nanotube wall to generate fluorescent ONT-AIST. (Figure 3)
 |
|
Fig. 3: Manufacturing process of fluorescent ONT-AIST.
|
(1) Specifically, place the specified amount of powder of an amphiphilic molecule and a fluorescent molecule in a beaker. (2) Add organic solution of the amount necessary to dissolve the molecules. Heat and stir the solution to dissolve the powder completely. (3) Leave the solution created in (2) for one night at room temperature, then the fluorescent molecules will be automatically embedded in the wall consisting of amphiphilic molecules in the self-assembly process. (Figure 4) This generates ONT-AIST with embedded fluorescent molecules, precipitated from the solution. (4) Filter the ONT-AIST, and then dry to produce fluorescent ONT-AIST powder.
 |
|
FIg. 4: Schematic diagram of the structure of fluorescent ONT-AIST.
|
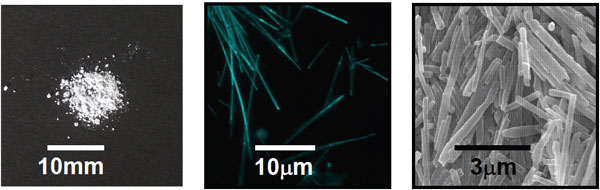
Fluorescent ONT-AIST was observed using a scanning electron microscope, demonstrating that the fluorescent molecules were embedded into the membrane of the tube wall of fluorescent ONT-AIST and the hollow structure remains unchanged. (Figure 5) And the ability of ONT-AIST to encapsulate molecules is expected to be kept.
 |
|
Fig. 5: Images of powder photograph (left), fluorescence microscopy (center), and scanning electron microscopy (right) of fluorescent ONT-AIST embedding pyrene into the nanotube membrane wall.
|
The simple procedure, just adding standard fluorescent molecules in the manufacturing process for ONT-AIST, makes it possible to manufacture fluorescent ONT-AIST of various colors at low cost and in a short period of time, with the ability to easily change the color.
Fluorescent ONT-AIST was produced in four colors as a trial (rhodamine B for red, rhodamine 6G for orange, fluorescein for yellow, and pyrene for blue). Each of these four fluorescent molecules is readily available at low cost. (Figure 6)
 |
|
Fig. 6: Emission of fluorescent ONT-AIST produced with rhodamine B (red), rhodamine 6G (orange), fluorescein (yellow), pyrene (blue).
|
Future Schedule
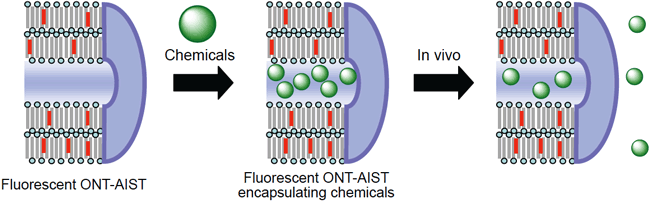
It is aimed to characterize the stability and behavior of ONT-AIST within an organism by observing cells to which ONT-AIST is transported. Also, as it is possible to observe in vivo transport behavior of ONT-AIST, study of DDS will be conducted in collaboration with a research group in the field of life science. (Figure 7)
 |
|
Fig. 7: Schematic diagram of chemicals encapsulation and release which utilize fluorescent ONT-AIST.
|
Currently, companies are being supplied with samples of ONT-AIST with a view to rapidly transfer the technology. In the future, applications from companies that wish to conduct further research with AIST and commercialize ONT-AIST will be accepted that allow further joint research to materialize.