Hideto Hoshino (Research Scientist) of the Cell Dynamics Research Group, the Research Institute for Cell Engineering (Director: Atsushi Miyake) of the National Institute of Advanced Industrial Science and Technology (President: Hiroyuki Yoshikawa) (hereinafter referred to as AIST), together with Yoshihiro Ohmiya (Leader) (currently a Professor, Graduate School of Medicine, Hokkaido University) and Yoshihiro Nakajima (Senior Research Scientist) of the research group have developed a technology for enhancing the efficacy of light emission from green fluorescent proteins (GFP) by using the luciferin - luciferase reaction, and a new bioluminescence imaging technique using the new technology.
Previously, an external light source was needed to yield the fluorescence of GFP in its various applications. On the other hand, bioluminescent organisms, such as fireflies, can glow autonomously by oxidizing a substrate called luciferin with an enzyme called luciferase. We have combined GFP with luciferase in developing the technology to produce auto-illuminating GFP that requires no external light source if luciferin is present. Different types of GFP will emit light of different colors. Furthermore, we have used this technology to develop a new bioluminescence imaging technique that permits the observation of a single cell.
The result of this study was published in the online edition of Nature Methods on July 8, 2007.
|
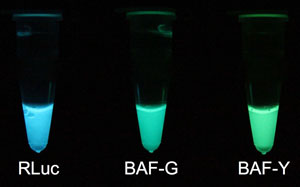
Fig. 1 In vitro luminescence on addition of luciferin
Left: RLuc natural blue light from Renilla reniformis prepared with Escherichia coli.
Center: Green light from BAF-G, an auto-illuminating fluorescent protein.
Right: Yellow-green light from BAF-Y, an auto-illuminating fluorescent protein
|
 |
“Photonics” is a fundamental technology for our daily lives, and various luminescence systems have been developed and are in use. In Japan, the phenomenon of bioluminescence has long been familiar because the Japanese enjoy the sight of fireflies flying; indeed, “Hotaru no Hikari” (The Light of the Firefly) is one of Japan’s most popular songs. The mechanism of this luminescence is gradually becoming understood. Bioluminescence is a unique, natural phenomenon that can be measured with high sensitivity and precision by simple operations. Further elucidation of the fundamental mechanisms involved in bioluminescence and the development of new systems based on the phenomenon are expected to occur.
AIST has examined the possibilities of bioluminescence and has developed it as a fundamental technology useful for human life. The Cell Dynamics Research Group has developed a tricolor luciferase technology based on beetle luciferase, and a technology based on secreted luciferase derived from Cypridina noctiluca.
Conventional GFP requires exposure to a light source, and the resulting fluorescence can only be measured in a laboratory by using complicated equipment, such as a fluorescent microscope, since it is necessary to exclude external light when measuring the emitted light. We focused on a phenomenon called bioluminescence resonance energy transfer (BRET), in which luciferin is used as the source of luminous energy, as occurs naturally in marine bioluminescent organisms such as Aequorea coerulescens and Renilla reniformis. We tried to develop a simple technique to make GFP glow and we applied this phenomenon in a bioluminescence imaging technique.
The study Development of a Single Cell Imaging Technique Using Bioluminescence was promoted as a Cell Dynamics Analysis Project by the New Energy and Industrial Technology Development Organization (NEDO).
As illustrated in Figure 2, we prepared an artificial protein in which GFP and luciferase derived from the marine bioluminescent organism Renilla reniformis are combined by means of an appropriate linker peptide, the sequence of which we optimized.
As a result, we achieved highly efficient BRET. We propose this as a new concept: BRET-based auto-illuminating fluorescent protein (BAF).
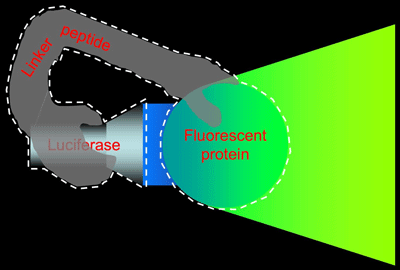 |
Fig. 2 BRET-based Auto-illuminating Fluorescent Protein (BAF)
BAF is an artificial protein in which luciferase and a fluorescent protein are bound together by a linker peptide (indicated by the white dashed line). Fluorescence is achieved by using the luciferase to illuminate the fluorescent protein.
|
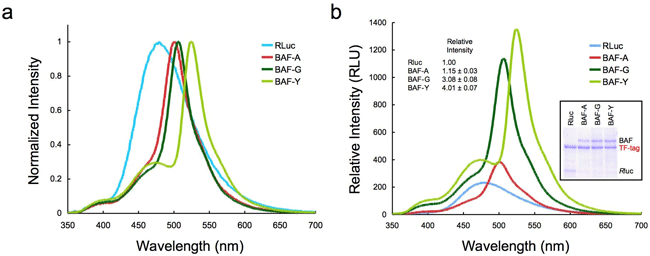
Three GFP variants ( AcGFP, E GFP, E YFP) were used to prepare artificial proteins, which we called BAF-A, BAF-G, and BAF-Y, respectively. These proteins glow only in the presence of luciferin (Fig. 1). The emission spectrum varies according to the characteristics of the particular GFP (Fig. 3a). Figure 3b shows that strengths of emissions from these artificial proteins per unit protein mass were four times stronger than those of normal luciferase. This may be because the emissions from the luciferase were amplified by the resonance energy transfer.
 |
|
Fig. 3 Emission spectra of normal luciferase (RLuc) and BAFs
(a) Spectra with the maximum values normalized to a standard value of 1. The normal spectrum (RLuc) is modified to reflect the characteristics of each GFP variant.
(b) Comparison of the emission intensities from equal masses protein measured under identical conditions. The light yield was calculated by measuring the area underneath each spectrum.
|
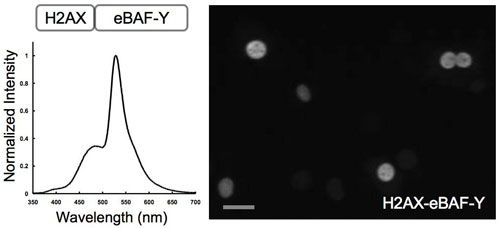
BAF-Y expressed in a cultured cell glowed efficiently inside a living cell. By using the newly developed bioluminescent microscope AB3000B Cellgraph (ATTO), we observed a cell containing an artificial protein comprising a protein distributed in chromatin of the cell nucleus (histone H2AX) fused with BAF-Y. As shown by Figure 4, single-cell bioluminescent imaging with high spatial and temporal resolution was achieved for the first time ever.
 |
|
Fig. 4 Single-cell bioluminescent imaging by using BAF-Y
The artificial protein H2AX-BAF-Y, is a fusion of the protein distributed in chromatin of the cell nucleus (histone H2AX) and BAF-Y, introduced into the cell.
Left: The bioluminescence spectrum of a living cell
Right: Six nuclei can be independently observed in this image. The dark portions in the nuclei are believed to correspond to be nucleolar region.
|
In this study, we showed that a combination of GFP variants and luciferase variants can be used to improving the brightness of BAF as bioluminescent probe in the future. We are going to modify the BAF itself, and try to understand the molecular mechanisms of efficient BRET to obtain an improved bioluminescent imaging system capable of being monitored in real time. We will also pursue other possibilities relating to this handy reagent.